Fiduxosin
Fiduxosin hydrochloride, 208992-74-9, NCGC00162178-02, AC1L58WW,
A-185980.1,ABT-980,UNII-W9O92HYT6I
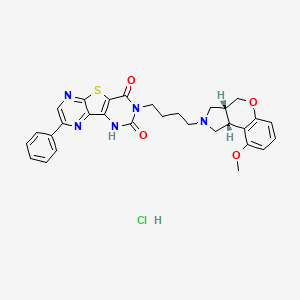
Molecular Formula: C30H30ClN5O4S
Molecular Weight: 592.1083
5-{4-[(2R,6R)-13-methoxy-8-oxa-4-azatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]butyl}-12-phenyl-8-thia-3,5,10,13-tetraazatricyclo[7.4.0.02,7]trideca-1(13),2(7),9,11-tetraene-4,6-dione hydrochloride
3-[4-((3aR,9bR)-cis-9-Methoxy-1,2,3,3a,4,9b-hexahydro-[1]-benzopyrano[3,4-c]pyrrol-2-yl)butyl]-8-phenyl-pyrazino[2',3':4,5]thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione
5-{4-[(2R,6R)-13-methoxy-8-oxa-4-azatricyclo[7.4.0.02,6]trideca-1(9),10,12-trien-4-yl]butyl}-12-phenyl-8-thia-3,5,10,13-tetraazatricyclo[7.4.0.02,7]trideca-1(13),2(7),9,11-tetraene-4,6-dione hydrochloride
Fiduxosin hydrochloride has been shown to be an α1-Adrenoceptor antagonist.
CAS NO
208992-74-9 Hydrochloride
208993-54-8 (free base)
208993-54-8 (free base)
Benign Prostatic Hyperplasia Therapy
Abbott Laboratories..INNOVATOR
WO 1998024791
Fiduxosin is an alpha(1)-adrenoceptor antagonist with higher affinity for alpha(1A)-adrenoceptors and for alpha(1D)-adrenoceptors than for alpha(1B)-adrenoceptors. Our hypothesis is that such a compound with higher affinity for subtypes implicated in the control of lower urinary tract function and lower affinity for a subtype implicated in the control of arterial pressure could result in a superior clinical profile for the treatment of lower urinary tract symptoms suggestive of benign prostatic obstruction.
Benign prostatic hyperplasia (BPH) is the most common cause of voiding dysfunction in middleaged and elderly males. [1] The prevalence of BPH increases with age. Epidemiological data indicate that the incidence of histological BPH is as high as 50% in men aged 60 years, rising to 88% in men aged 80 years.
Clinical data suggest that the use of a selective1A-adrenoceptor antagonist results in clinical benefit. Fiduxosin is a novel orally active agent that is a selective1A-adrenoceptor antagonist. Since the intended use of fiduxosin is in a middle-aged/elderly male population, the pharmacokinetics of single doses of fiduxosin were evaluated in a first-in-human study conducted using healthy elderly (≥60 years) male volunteers.
 Fiduxosin
Fiduxosin
............................................................................
SYNTHESIS
PATENT EP0942911B1
-
-
-
-
-
-
- Example 108
- 3-[4-((3aR,9bR)-cis -9-Methoxy-1,2,3,3a,4,9b-hexahydro-[1]-benzopyrano[3,4-c]pyrrol-2-yl)butyl]-8-(4-hydroxyphenyl)-pyrazino[2',3':4,5]thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione
- The product of Example 16 (0.07 g,0.105 mmol) and 4-(methoxymethyloxy) phenyl boronic acid (0.02 g, 0.11 mmol) prepared by the procedure described in Tetr.Lett., 31, 27, (1990) were treated as described in Example 106 to yield 0.029g(45%) of MOM-protected product. To the solution of this product (0.11g, 0.17 mmol) in CH3OH/THF was added 2N HCl (0.2ml) and the reaction mixture was refluxed for 1 hour. The reaction was evaporated and partitioned in NaHCO3 sol. and CH2Cl2/CH3OH to yield 0.005 g (51%) of the title compound.
- 1H NMR (500 MHz, CDCl3) d 1.81 (m, 2H), 1.98 (m, 2H), 2.25 (m, 1H), 2.65 (m, 1H), 2.88 (m, 1H), 3.08 (m, 2H), 3.22(m, 2H), 3.65 (m, 1H), 3.73 (m, 1H), 3.82 (s, 3H), 3.9 (m, 1H), 4.25 (m, 1H), 4.42 (m, 1H), 6.52 (m, 2H), 7.38 (m, 2H),7.49(m, 1H), 7.9 (t, 1H), 8.09 (d, 1H),9.12 (s, 1H);
- MS(ESI)m/e 572 (M+H)+.
Example 16
3-[4-((3aR,9bR)- cis -9-Methoxy-1,2,3,3a,4,9b-hexahydro-[1]-benzopyrano[3,4-c]pyrrol-2-yl)butyl]-8-chloro-pyrazino[2',3':4,5]thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione hydrochloride
- The product from Example 10 C (0.27 g, 1.0 mmol) and the product from Example 1E (0.20 g, 0.73 mmol) were treated as described in Example 1F to yield 0.29 g (77%) of the title compound: m.p. 220-222°;
- 1H NMR (300 MHz, CDCl3(free base)) δ 8.68 (s, 1H), 7.0 (t, 1H), 6.48 (d, 1H), 6.45 (d, 1H), 4.28 (m, 1H), 4.12 (m, 3H), 4.0 (m, 2H), 3.75 (s, 3H), 3.6 (m, 1H), 3.08 (m, 3H), 2.9 (m, 2H), 1.75 (m, 4H); MS (DCI/NH3) m/e 514(M+H)+;
- Analysis calc'd for C24H24ClN5O4S·HCl·0.75H2O: C, 51.11; H, 4.74; N, 12.42; found: C, 51.09; H, 4.75; N, 12.43.
..........................
SYNTHESIS

Fiduxosin
Fiduxosin (ABT-980), α1a-adrenoreceptor antagonist, a development compound at Abbot for the treatment of benign prostate hyperplasia, is disclosed in Organic Process Research & Development 2004, 8, 897-902 and references cited therein.
Org. Proc. Res. Dev., 2004, 8 (6), pp 897–902
DOI: 10.1021/op049889k
The synthetic route for preparation of Fiduxosin is as follows:

Fiduxosin (1) has been under development at Abbott Laboratories for the treatment of benign prostatic hyperplasia. A convergent strategy required methodologies for preparation of an enantiomerically pure 3,4-cis-disubstituted pyrrolidine and a 2,3,5-trisubstituted thienopyrazine in a regiospecific manner.
A [3+2] cycloaddition of an enantiopure azomethine ylide followed by a diastereoselective crystallization was employed to prepare the benzopyranopyrrolidine in high diastereomeric and enantiomeric purity. Conditions for reduction of an O-aryl lactone susceptible to epimerization were developed, and cyclization of the alcohol/phenol to the ether was accomplished in high yield.
The thienopyrazine was prepared by condensation of methyl thioglycolate and a regiospecifically prepared 2-bromo-3-cyano-5-phenylpyrazine. Conditions for effective halogen substitutive deamination to prepare regiospecific trisubstituted pyrazines will be described.
The mixture of 5 - and 6-phenyl regioisomers of 2-hydroxy-3-carboxamidopyrazine (IX) and (X), prepared by a known method, was treated with POCl3 and Et3N to produce the corresponding chloro nitriles (XI) and (XII ). Condensation of this mixture with methyl thioglycolate in the presence of NaOMe, followed by chromatographic separation of isomers furnished the desired thienopyrazine intermediate (XIII).
...........................................................
 Fiduxosin
Fiduxosin
................................................................
SYNTHESIS

Cycloaddition of the azomethine ylide resulting from N-trimethylsilylmethyl-N-methoxymethyl-(R)-alpha-methylbenzylamine (II) to 5-methoxycoumarin (I) produced the chiral cis-benzopyranopyrrole system (III). Lactone reduction by means of LiAlH4 or LiBH4 afforded diol (IV). After conversion of the primary alcohol of (IV) to either the corresponding chloride or the mesylate, cyclization in the presence of potassium tert-butoxide generated the tricyclic compound (V).
The alpha-methylbenzyl group of ( V) was removed by catalytic hydrogenation to give amine (VI), which was alkylated with 4-bromobutyronitrile yielding (VII). Reduction of the cyano group of (VII) using LiAlH4 in the presence AlCl3 or by catalytic hydrogenation in the presence of Raney -Ni produced the primary amine (VIII).

.........................................................

The mixture of 5 - and 6-phenyl regioisomers of 2-hydroxy-3-carboxamidopyrazine (IX) and (X), prepared by a known method, was treated with POCl3 and Et3N to produce the corresponding chloro nitriles (XI) and (XII ). Condensation of this mixture with methyl thioglycolate in the presence of NaOMe, followed by chromatographic separation of isomers furnished the desired thienopyrazine intermediate (XIII).

...................................................................

In a regioselective synthetic method, phenyl glyoxime (XIV) was condensed with aminomalononitrile to produce the pyrazine N-oxide (XV). Reduction of the N-oxide of (XV) with triethyl phosphite yielded (XVI). Diazotization of the amino group of (XVI), followed by diazo displacement with CuBr2, furnished bromo pyrazine (XVII). This was then cyclized with methyl thioglycolate as above to yield the desired thienopyrazine intermediate (XIII).

..........................................................

In an alternative synthesis, phenylacetaldehyde (XVIII) was condensed with pyrrolidine (XIX) to give enamine (XX). Nitrosation of malononitrile (XXI), followed by treatment with tosyl chloride, produced the O-tosyl oxime (XXII). This was condensed with enamine (XX), and to the intermediate adduct (XXIII) was added thiophenol producing the phenylthiopyrazine (XXIV). Subsequent oxidation of the sulfide group of (XXIV) to sulfone (XXV), followed by condensation with methyl thioglycolate, gave the desired thienopyrazine (XIII).

.......................................................................

The amino ester intermediate (XIII) was treated with phosgene and Et3N, and to the resulting isocyanate (XXVI) was added the primary amine (VIII), producing urea (XXVII). Then, cyclization of (XXVII) in refluxing toluene generated the desired compound,
fiduxosin
| 11805208 | 2-1-2002 | Effect of fiduxosin, an antagonist selective for alpha(1A)- and alpha(1D)-adrenoceptors, on intraurethral and arterial pressure responses in conscious dogs. | The Journal of pharmacology and experimental therapeutics |
| 11805209 | 2-1-2002 | Modeling of relationships between pharmacokinetics and blockade of agonist-induced elevation of intraurethral pressure and mean arterial pressure in conscious dogs treated with alpha(1)-adrenoceptor antagonists. | The Journal of pharmacology and experimental therapeutics |
| 11996327 | 1-1-2002 | Effect of food on the pharmacokinetics of fiduxosin in healthy male subjects. | European journal of drug metabolism and pharmacokinetics |
| 22740655 | 9-1-2012 | Identification and analysis of hepatitis C virus NS3 helicase inhibitors using nucleic acid binding assays. | Nucleic acids research |
| 22252091 | 3-1-2012 | Small molecule screening identifies targetable zebrafish pigmentation pathways. | Pigment cell & melanoma research |
| 20427476 | 7-1-2010 | A small molecule inverse agonist for the human thyroid-stimulating hormone receptor. | Endocrinology |
| 19583963 | 11-1-2009 | A new homogeneous high-throughput screening assay for profiling compound activity on the human ether-a-go-go-related gene channel. | Analytical biochemistry |
| 19734910 | 10-1-2009 | Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. | Nature chemical biology |
| 17417631 | 5-1-2007 | Chemical genetics reveals a complex functional ground state of neural stem cells. | Nature chemical biology |
| 16604538 | 5-1-2006 | Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology |
| 12017348 | 5-1-2002 | Single- and multiple-dose pharmacokinetics of fiduxosin under nonfasting conditions in healthy male subjects. | Journal of clinical pharmacology |
| 12005359 | 5-1-2002 | Multiple dose pharmacokinetics of fiduxosin under fasting conditions in healthy elderly male subjects. | The Journal of pharmacy and pharmacology |
| 11805207 | 2-1-2002 | Preclinical pharmacology of fiduxosin, a novel alpha(1)-adrenoceptor antagonist with uroselective properties. | The Journal of pharmacology and experimental therapeutics |









No comments:
Post a Comment