Azadirachtin
11141-17-6 cas no
Azadirachtin, a chemical compound belonging to the limonoid group, is asecondary metabolite present in neem seeds. It is a highly oxidizedtetranortriterpenoid which boasts a plethora of oxygen functionality, comprising an enol ether, acetal, hemiacetal, and tetra-substituted oxirane as well as a variety of carboxylic esters.
This compound is a tetraterpenoid characteristic of the Meliaceae family but particularly from the Neem tree (A. indica), indigenous to India. The compound is found in bark, leaves and fruits of the tree but seeds have the highest concentration. This compound has not yet been synthesized in the laboratory, but when isolated and tested pure the results have been less than when extracts are used. In the extract 18 compounds have been identified among which salanine, meliantrol and azadiractin are most prominent, the latter being in the highest concentration. Azadirachtin shows antifeedant activity, is a growth regulator, inhibits oviposition and is also a sterilizing compound. Today, commercial formulations of neem may be found with names like Neem Gold, Neemazal, Econeem, Neemark, Neemcure and Azatin among others, in many countries including the United States, India, Germany and several Latin American countries.
Azadirachtin has a complex molecular structure, and as a result the first synthesis was not published for over 22 years after the compound's discovery. The first total synthesis was completed by Steven Ley in 2007.[1][2] Both secondary and tertiary hydroxyl groups and tetrahydrofuran ether are present and the molecular structure reveals 16 stereogenic centres, 7 of which are tetrasubstituted. These characteristics explain the great difficulty encountered when trying to produce it by a synthetic approach. The described synthesis was actually a relay approach, with the heavily functionalized decalin being made by total synthesis in a small scale but also being derived from the natural product itself for the purpose of obtaining gram amounts of the material to complete the synthesis.
"I would rank this as being one of the very toughest syntheses so far reported."
- Steven Ley, University of Cambridge, UK, 1 G E Veitch et al, Angewandte Chemie, 2007, DOIs: 10.1002/anie.200703027 and 10.1002/anie.200703028
2 M L Maddess et al, Angewandte Chemie, 2006, 46, 591 (DOI: 10.1002/anie.200604053)
- Steven Ley, University of Cambridge, UK, 1 G E Veitch et al, Angewandte Chemie, 2007, DOIs: 10.1002/anie.200703027 and 10.1002/anie.200703028
2 M L Maddess et al, Angewandte Chemie, 2006, 46, 591 (DOI: 10.1002/anie.200604053)
IT TOOK 22 YEARS and the efforts of more than 40 chemists, but Steven V. Ley's group has finally managed to complete a 64-step synthesis of azadirachtin, a naturally occurring insecticide (Angew. Chem. Int. Ed., DOI:10.1002/anie.200703027 and 10.1002/anie.200703028).

Isolated from the Indian neem tree Azadirachta indica, azadirachtin possesses a small but densely functionalized architecture. It has 16 stereogenic centers, seven of which are tetrasubstituted, and a diverse array of oxygenated functionalities.
"This has been a tough project from start to finish, as the molecule is so prone to rearrangement under acidic, basic, or photolytic conditions," says Ley, a chemistry professor at England's Cambridge University. "It has forced us to be inventive."
Of the many hurdles the researchers had to overcome, Ley reckons the most challenging was coupling the molecule's two main fragments. After years of attempts at this convergent approach, the group was finally able to marry these two fragments by means of a propargylic enol ether Claisen reaction. The next step, a radical-induced cyclization, elegantly constructed one sterically dense portion of the molecule.
"Making a molecule such as this is not an Everest-climbing exercise; it's about what you learn from the experience," Ley says. "We can be proud of the new methods and solutions to the tough problems we have encountered, and now we have the tools and procedures to really work out how this molecule functions biologically."
Amos B. Smith III, a chemistry professor at the University of Pennsylvania, calls the work "an outstanding achievement, further demonstrating Ley and colleagues as superb tacticians in the art of complex-molecule total synthesis."
Smith adds: "More important than the actual conquest is the exciting new chemistry that has emanated over the past 22 years from the Ley and other laboratories who have participated in this monumental challenge. Clearly, the science of synthesis is the winner."
It was initially found to be active as a feeding inhibitor towards the desert locust(Schistocerca gregaria),[3] it is now known to affect over 200 species of insect, by acting mainly as an antifeedant and growth disruptor, and as such it possesses considerable toxicity toward insects (LD50(S. littoralis): 15 μg/g). It fulfills many of the criteria needed for a natural insecticide if it is to replace synthetic compounds. Azadirachtin is biodegradable (it degrades within 100 hours when exposed to light and water) and shows very low toxicity to mammals(the LD50 in rats is > 3,540 mg/kg making it practically non-toxic).
This compound is found in the seeds (0.2 to 0.8 percent by weight) of the neemtree, Azadirachta indica (hence the prefix aza does not imply an aza compound, but refers to the scientific species name). Many more compounds, related to azadirachtin, are present in the seeds as well as in the leaves and the bark of the neem tree which also show strong biological activities among various pest insects [4][5] Effects of these preparations on beneficial arthropods are generally considered to be minimal. Some laboratory and field studies have found neem extracts to be compatible with biological control. Because pure neem oil contains other insecticidal and fungicidal compounds in additional to azadirachtin, it is generally mixed at a rate of 1 ounce per gallon (7.8 ml/l) of water when used as a pesticide.
Azadirachtin is formed via an elaborate biosynthetic pathway, but is believed that the steroid tirucallol is the precursor to the neem triterpenoid secondary metabolites. Tirucallol is formed from two units of farnesyl diphosphate (FPP) to form a C30 triterpene, but then loses three methyl groups to become a C27steroid. Tirucallol undergoes an allylic isomerization to form butyrospermol, which is then oxidized. The oxidized butyrospermol subsequently rearranges via a Wagner-Meerwein 1,2-methyl shift to form apotirucallol.
Apotirucallol becomes a tetranortriterpenoid when the four terminal carbons from the side chain are cleaved off. The remaining carbons on the side chain cyclize to form a furan ring and the molecule is oxidized further to form azadirone and azadiradione. The third ring is then opened and oxidized to form the C-seco-limonoids such as nimbin, nimbidinin and salannin, which has been esterified with a molecule of tiglic acid, which is derived from L-isoleucine. It is currently proposed that the target molecule is arrived at by biosynthetically converting azadirone into salanin, which is then heavily oxidized and cyclized to reach azadirachtin.
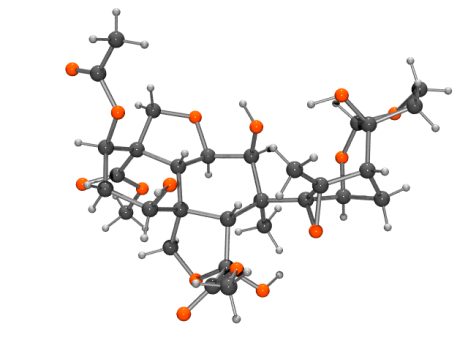
Azadirachtin ball and stick view
- Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV (2007). "Synthesis of azadirachtin: a long but successful journey". Angew. Chem. Int. Ed. Engl. 46 (40): 7629–32.doi:10.1002/anie.200703027. PMID 17665403.
- Sanderson K (August 2007). "Chemists synthesize a natural-born killer". Nature 448 (7154): 630–1. doi:10.1038/448630a.PMID 17687288.
- Butterworth, J; Morgan, E (1968). "Isolation of a Substance that suppresses Feeding in Locusts". Chemical Communications (London) (1): 23.doi:10.1039/C19680000023.
- Senthil-Nathan, S., Kalaivani, K., Murugan, K., Chung, G. (2005). "The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guenée) the rice leaffolder".Pesticide Biochemistry and Physiology 81 (2): 113.doi:10.1016/j.pestbp.2004.10.004.
- Senthil-Nathan, S., Kalaivani, K., Murugan, K., Chung, P.G. (2005). "Effects of neem limonoids on malarial vector Anopheles stephensi Liston (Diptera: Culicidae)". Acta Tropica96 (1): 47. doi:10.1016/j.actatropica.2005.07.002.PMID 16112073.
Extracts of the neem tree (Azadirachta indica) , the chinaberry tree (Melia azedarach) , and other plants in the family Meliaceae (e.g. Azadirachta excelsa) have long been known to have insectici al activity (Natural Pesticides from the Neem Tree, Proc. 1st Int'l Neem Conf. 1980 [H. Schmutterer et al. eds. 1982]; Natural Pesticides from the Neem Tree and Other Tropical Plants, Proc. 2nd Int'l Neem Conf. 1983 [H. Schmutterer and K.R.S. Asher eds. 1984]; Natural Pesticides from the Neem Tree and Other Tropical Plants, Proc. 3rd Int'l Neem Conf. 1986 [H. Schmutterer and K.R.S. Asher eds. 1987]). Azadirachtin, a major active ingredient in many of these extracts, is a limonoid of the tetranortriterpenoid type. Azadirachtin has been shown to be a potent insect growth regulator and feeding deterrent with value as an active ingredient in commercial insecticides (R.B. Yamasaki et al. (1987) J. Agric. Food Che . 3_5:467-471) .
The use of azadirachtin in commercial insecticides requires that the compound be extracted from the plant material and concentrated in a convenient form. Suitableazadirachtin concentrates are solid in form, have high levels of azadirachtin
(typically greater than 1-3% by weight) , and contain little or no plant oils and water. These characteristics facilitate handling, storage, distribution, and formulation.
Various methods of extracting azadirachtin from neem seeds are known in the art. J.H. Butterworth and E.D. Morgan, (1971) J. Insect. Physiol. Y]_ 969-911, describe the extraction of azadirachtin and neem oil from neem seeds using ethanol, a common solvent for azadirachtin isolation. Further separation steps (including filtration, partition of the soluble portion between light petroleum and methanol, and chromatography of the methanol extract on Floridin earth) are used to concentrate the azadirachtin to obtain a solid. Larson (U.S. Patent No. 4,556,562) discloses a similar method whereby neem seeds are first reduced in size to that of a regular grind of coffee. Azadirachtin is then extracted at elevated temperatures, again using ethanol. No further concentration steps are used, however, and the final extract is a liquid containing only 0.5-1% azadirachtin. Walter (U.S. Patent No. 4,946,681) describes a related process for extractingazadirachtin from ground de-oiled neem seeds with aprotic solvents or alcohols. The liquid extract is further treated with molecular sieves to remove water. Carter et al. (U.S. Patent No. 5,001,146) describes a two-step extraction process comprising a "cleanup" extraction with a non- polar aprotic solvent to remove the neem oil from the seeds, followed by a second extraction of the defatted neem seeds using a more polar, azadirachtin-soluble solvent. The final extract from this process is a solution containing about 4.5 g/1 azadirachtin. Kleeberg (WO 92/16109) also describes a multi-step procedure whereby the azadirachtin is first extracted from neem oil and plant material using water. The aqueous fraction is then extracted with an organic solvent to remove the azadirachtin from the water. A solid azadirachtin concentrate is ultimately obtained using a slow and inefficient phase separation followed by concentration of the organic phase and crystallization.
Inefficiencies and limitations associated with the processes described above illustrate the need for more practical and versatile methods for obtainingazadirachtin concentrates. Specifically, current methods that produce extracts containing suitably high azadirachtin content (>l-3% by weight) all involve expensive steps to remove neem oil, water, and other impurities. Such steps include decantation, phase separation, chromatography, crystallization, treatment with molecular sieves, and other purification steps. None of the known methods provides a suitably efficient, economical, or practical means for recoveringazadirachtin in a concentrated solid form that contains little or no neem oil or water.
It is known that the seeds and other parts of the neem tree [Azaderachta indicia and related species) contain natural pesticidal compositions. The main active pesticidal composition is azadirachtin which is a tetranortriterpenoid that causes feeding inhibition and growth inhibition in a variety of organisms including insects, mites and nematodes. It is possible that there are a number of similar insecticidal compounds present in neem extracts that partition with the azadirachtin. As used in this specification, the term azadirachtin is taken to include all insecticidal terpenoids present in neem extracts that partition with azadirachtin. In recoveringazadirachtin from neem seeds it is necessary to separate the active constituent from other materials including the other triterpenes, the oil. fibre and other insoluble materials, and water soluble constituents such as sugars and water soluble proteins. These separation procedures are complicated by the fact thatazadirachtin is susceptible to hydrolysis in water and to heat degradation.
One conventional method for extracting azadirachtin from neem seeds involves the use of three organic solvents and two liquid/liquid partition steps. Firstly, the neem seeds are pressed to remove the majority of the neem oil. The resulting expeller cake is then extracted with methanol. The methanol extracts a wide range of substances including azadirachtin and the other triterpenes, diterpenes. the residual oil, and some polysaccharides and proteins. To produce a powder from the methanolic supernatant, the extract must undergo a number of clean-up steps.
In the first clean-up step, the supernatant is concentrated and partitioned against hexane, or a similar non-polar solvent, to remove the oils and diterpenes. The hexane is then driven off in a still and the resulting oil is collected. The second clean-up step involves taking the de-oiled supernatant and driving off the methanol. The resulting tar is then resolved in ethyl acetate, or a similar solvent, and partitioned against water to remove the polysaccharides and water soluble proteins. The ethyl acetate which contains the azadirachtin and other triterpenes is then evaporated to produce an azadirachtin rich powder.
There are a number of problems associated with this conventional method, with the major problem being cross-contamination of the solvents used in the process and the resulting variation in product quality. While good quality azadirachtinpowder can be produced when first using the process with fresh solvents, after a number of cycles problems with cross- contamination do arise. While hexane and methanol are normally considered essentially immiscible, in a multi-component system, such as is created during the extraction of azadirachtin described above, the hexane and methanol do demonstrate some miscibility. The change in the polarity of the solvents allows oils to be carried through the hexane partition and so creates difficulties in producing a non-oily or flowable powder. A more serious problem is any occurrence of cross-contamination between the methanol and ethyl acetate. If the methanol is not removed in the drying step after the hexane partition, cross-contamination with ethyl acetate occurs. This contamination of the ethyl acetate with methanol causes azadirachtin to be carried over into the water in the ethyl acetate/water partition or in the worst case prevents a partition forming at all. A further problem with the conventional method for extractingazadirachtin is the production of waste water with a high biological oxygen demand (BOD). In many countries, the release of waste water with high BOD is not permitted and requires the installation of a relatively expensive water treatment facility. An alternative azadirachtin extraction process is described in Australian patent no 661482 to Trifolio-M GmbH, Herstellung Und Vertrieb Hochreiner Biosubstanzen. In this alternative process, the neem seed is pressed or crushed to remove the majority of the oil and the expeller cake is extracted with warm water. The warm water extraction removes the azadirachtin. some of the more polar triterpenes. the majority of the polysaccharides and water soluble proteins, and a slight amount of the more polar oily compounds. The aqueous supernatant is partitioned against a solvent of intermediate polarity, such as ethyl acetate or dichloro me thane. with the azadirachtin partitioning into the organic layer. The organic layer can then be concentrated under vacuum and theazadirachtin precipitated through the addition of a non-polar solvent, such as hexane or petroleum ether. While high yields of azadirachtin powder are produced using this alternative process, the process still results in the discharge of an aqueous solution loaded with polysaccharides and proteins which, in some countries, will require treatment to meet environmental standards. Further, the precipitation step where a non-polar solvent is added to the concentrated supernatant results in cross-contamination with its attendant problems and expense of requiring purification of the solvents prior to their re-use in the extraction process.
G. E. Veitch, E. Beckmann, B. J. Burke, A. Boyer, S. L. Maslen, S. V. Ley*
University of Cambridge, UK
Synthesis of Azadirachtin: A Long but Successful Journey
Angew. Chem. Int. Ed. 2007, 46: 7629-7632
University of Cambridge, UK
Synthesis of Azadirachtin: A Long but Successful Journey
Angew. Chem. Int. Ed. 2007, 46: 7629-7632
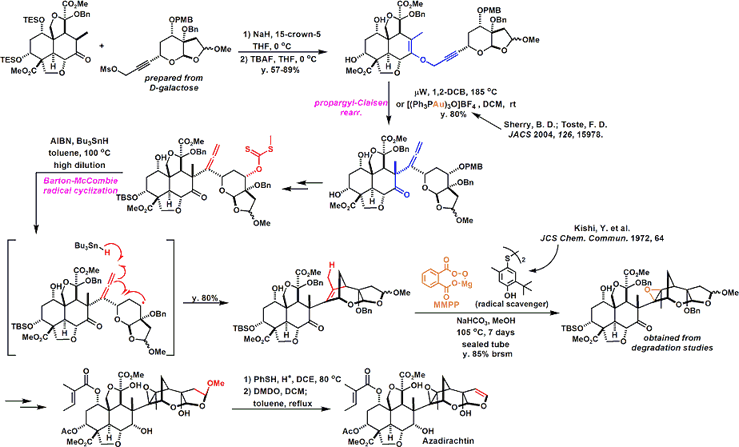
Azadirachtin is an insect antifeedant. A major challenge in this 22-year odyssey was the construction of 16 contiguous stereogenic centers seven of which are quaternary. The excerpt depicted here focuses on the Claisen rearrangment used to construct the congested C8-C14 bond.
Compounds A and G were obtained by total synthesis and by degradation of azadirachtin. The degradation route enabled exploration of the difficult closing stages of the synthesis. Note the harsh conditions required to effect the difficult epoxidation F→G.
......................................
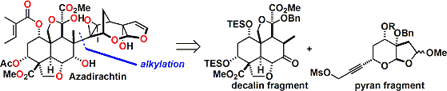
The basic skeleton of the decalin fragment, intramolecularDiels-Alder reaction and aldol type cyclization and is building well using. Together is important for the selectivity of the Diels-Alder reaction, a silyl group in the after Tamao-Fleming oxidationhas become a stepping stone for introducing a hydroxyl group by.
Subsequently, a fragment coupling. An attempt was made to make the binding by alkylation, but what has been obtained, It was a compound the reaction is going on in the oxygen atom of the enol.
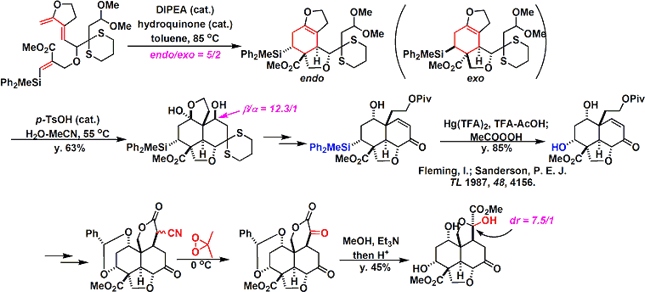
Anyway Since make a bond between the fragments, was a stepping stone to this Claisen rearrangement by carbon - and is trying to build a carbon bond. Reaction seems to have gone even heat, but has been developed by Toste et al (I) catalyst Au Claisen rearrangement using that seems to have been effective.
Conversion fairly high hurdle is followed thereafter. Barton-McCombie conditions and it has succeeded in building carbon skeleton of the right half be subjected to a radical cyclization reaction by. At a position that is crowded Then epoxidation and we have been. Becomes the conditions investigated thoroughly that (7 days) radical scavenger addition temperature (100 ℃ or more) time, it's easy to imagine that even just one step here, consider mind-boggling are stacked . Same compound obtained in Degradation Studies This is obtained, advances the synthesized according to the reverse route later, we have completed the synthesis of azadirachtin.
Conversion fairly high hurdle is followed thereafter. Barton-McCombie conditions and it has succeeded in building carbon skeleton of the right half be subjected to a radical cyclization reaction by. At a position that is crowded Then epoxidation and we have been. Becomes the conditions investigated thoroughly that (7 days) radical scavenger addition temperature (100 ℃ or more) time, it's easy to imagine that even just one step here, consider mind-boggling are stacked . Same compound obtained in Degradation Studies This is obtained, advances the synthesized according to the reverse route later, we have completed the synthesis of azadirachtin.
Total synthesis route of the 64 steps that have been achieved
Synthesis of Azadirachtin: A Long but Successful Journey Veitch, GE; Beckmann, E.; Burke, BJ; Boyer, A.; Maslen, SL; Ley, SV .... Angew Chem Int Ed 2007 , 46 ., 7629 DOI:10.1002/Anie.200703027A Relay Route for the Synthesis of Azadirachtin Veitch, GE; Beckmann, E.; Burke, B. j;. Boyer, A.; Ayats, C.; Ley, SV ... Angew Chem Int Ed. 2007 , 46 ., 7633 DOI: 10.1002/Anie.200703027


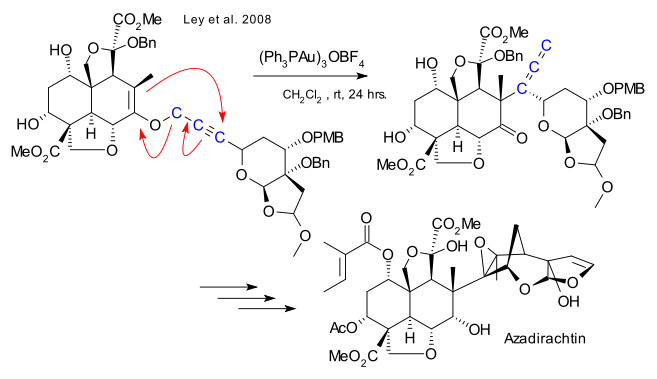
12-Ethoxynimbolinin B is a limonoid compound. Limonoids are a class of tetranortriterpenoids with structural diversity and wide range of bioactivities, including insect antifeedant activity, and antimicrobial, antiprotozoal, anti-inflammatory, and anticancer activities. 12-Ethoxynimbolinin B
ReplyDelete