
CAS No.......518048-05-0 (free acid)
871038-72-1 (monopotassium salt)IUPAC Name:- N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)
871038-72-1 (monopotassium salt)IUPAC Name:- N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)
Organic Process Research and Development, 2011 , vol. 15, 1 pg. 73 - 83,
143 - 144.1 °C(free acid)
MW: 444.42
..........................................................
K SALT
C20H20FN6O5*K, 482.513
MP..275 - 277 °C
European Journal of Medicinal Chemistry, 2012 , vol. 50, pG. 361 - 369
Drug information:- Raltegravir is an Anti-microbial drug further classified as anti-viral agent of the class integrase inhibitor. It is used either signally or in combination with other drugs for the treatment of human immunodeficiency virus (HIV) and further clinical trials are in process.
Raltegravir (RAL, Isentress, formerly MK-0518) is an antiretroviral drug produced by Merck & Co., used to treat HIV infection.[1] It received approval by the U.S. Food and Drug Administration (FDA) on 12 October 2007, the first of a new class of HIV drugs, the integrase inhibitors, to receive such approval.[2][3]
In December 2011, it received FDA approval for pediatric use in patients ages 2–18, taken in pill form orally twice a day by prescription with two other antiretroviral medications to form the cocktail (most anti-HIV drugs regimens for adults and children use these cocktails). Raltegravir is available in chewable form but- because the two tablet formulations are not interchangeable- the chewable pills are only approved for use in children two to 11. Older adolescents will use the adult formulation.[4]
Raltegravir targets integrase, an HIV enzyme that integrates the viral genetic material into human chromosomes, a critical step in the pathogenesis of HIV. The drug is metabolized away via glucuronidation.[5]
Raltegravir is taken orally twice daily.[3] Doses of 200, 400, and 600 mg have been studied.
At the 2007 Conference on Retroviruses and Opportunistic Infections, researchers presented Phase III data showing that 77% of patients taking the 400 mg dose of raltegravir plus other antiretroviral drugs reached HIV viral loads below 400 copies, nearly twice as many compared with a control group.
Raltegravir was initially approved only for use in individuals whose infection has proven resistant to otherHAART drugs.[3] However, in July 2009, the FDA granted expanded approval for Raltegravir for use in all patients.[6] As with any HAART medication, raltegravir is unlikely to show durability if used as monotherapy.
In a study of the drug as part of combination therapy, raltegravir exhibited potent and durable antiretroviral activity similar to that of efavirenz at 24 and 48 weeks but achieved HIV-1 RNA levels below detection at a more rapid rate. After 24 and 48 weeks of treatment, raltegravir did not result in increased serum levels of total cholesterol, low-density lipoprotein cholesterol, or triglycerides.[7][8]
Raltegravir significantly alters HIV viral dynamics and decay and further research in this area is ongoing. In clinical trials patients taking raltegravir achieved viral loads less than 50 copies per millitre sooner than those taking similarly potent Non-nucleoside Reverse Transcriptase Inhibitors orProtease Inhibitors. This statistically significant difference in viral load reduction has caused some HIV researchers to begin questioning long held paradigms about HIV viral dynamics and decay.[9] Research into raltegravir's ability to affect latent viral reservoirs and possibly aid in the eradication of HIV is currently ongoing.[10]
Research results were published in the New England Journal of Medicine on July 24, 2008. The authors concluded that "raltegravir plus optimized background therapy provided better viral suppression than optimized background therapy alone for at least 48 weeks." [11]
Research on human cytomegalovirus (HCMV) terminase proteins demonstrated that Raltegravir may block viral replication of the herpesviruses.[12]
In January 2013, a Phase II trial was initiated to evaluate the therapeutic benefit of raltegravir in treating multiple sclerosis (MS).[13] The drug is active against Human Endogenous Retroviruses(HERVs) and possibly Epstein-Barr Virus, which have been suggested in the pathogenesis of relapsing-remitting MS.
Raltegravir was generally well tolerated when used in combination with optimized background therapy regimens in treatment-experienced patients with HIV-1 infection in trials of up to 48 weeks' duration.[14]
Synthesis

WO 2006060730
.........................................................
- Savarino A (December 2006). "A historical sketch of the discovery and development of HIV-1 integrase inhibitors". Expert Opin Investig Drugs 15 (12): 1507–22. doi:10.1517/13543784.15.12.1507.PMID 17107277.
- "FDA approval of Isentress (raltegravir)". U.S. Food and Drug Administration (FDA). June 25, 2009. Retrieved 2009-11-15.
- "Isentress Drug Approval Package". U.S. Food and Drug Administration (FDA). February 22, 2008. Retrieved 2009-11-15.
- http://www.everydayhealth.com/hiv-aids/1222/fda-okays-raltegravir-for-kids-teens-with-hiv.aspx?xid=aol_eh-hiv_6_20111219_&aolcat=HLT&icid=maing-grid7%7Cmain5%7Cdl10%7Csec3_lnk2%26pLid%3D122480
- HIV Antiretroviral Agents in Development
- "UPDATE 2-FDA OKs widened use of Merck's Isentress HIV drug". Reuters. 2009-07-10.
- Markowitz M, Nguyen BY, Gotuzzo E, et al. (2007). "Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study". J. Acquir. Immune Defic. Syndr. 46 (2): 125–33. doi:10.1097/QAI.0b013e318157131c. PMID 17721395.
- Stephenson J (2007). "Researchers buoyed by novel HIV drugs: will expand drug arsenal against resistant virus". JAMA 297 (14): 1535–6. doi:10.1001/jama.297.14.1535. PMID 17426263.
- Faster Viral Decay With Raltegravir
- ClinicalTrials.gov NCT00554398 Impact of MK-0518 (Raltegravir) Intensification on HIV-1 Viral Latency in Patients With Previous Complete Viral Suppression
- Steigbigel RT, Cooper DA, Kumar PN, et al. (July 2008). "Raltegravir with optimized background therapy for resistant HIV-1 infection". N. Engl. J. Med. 359 (4): 339–54.doi:10.1056/NEJMoa0708975. PMID 18650512.
- Drug against AIDS could be effective against herpesvirus
- Raltegravir (Isentress) Pilot Study in Relapsing Multiple Sclerosis (INSPIRE)
- Croxtall JD, Keam SJ. (2009). "Raltegravir". Drugs 69 (8): 1059–75. doi:10.2165/00003495-200969080-00007. PMID 19496631.
- Belyk, K. M.; Morrison, H. G.; Jones, P.; Summa, V.; 2007, WO 2006060730
- Manufacturer's website
- MK-0518 at Aidsmedscom[dead link]
- Integrase Inhibitor Raltegravir (MK-0518) Doubles HIV Suppression in Treatment-Experienced Patients (aidsmap 28 February 2007)
- RMK-0518 Abstract from CROI 2007
- Interim Results From Phase II Study Of MK-0518
- World patent covering the potassium salt
- Raltegravir Pharmacokinetics
...................................................................................................................................................................
Raltegravir, also referred to as Raltegravir free-hydroxy, N-(2-(4-(4-fluorobenzyl- carbamoyl)-5-hydroxy-l-methyl-6-oxo-l ,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl- l ,3,4-oxadiazole-2-carboxamide, having the following structure;
is an antiretroviral drug used to treat HIV infection. Raltegravir targets integrase, an HIV enzyme that integrates the viral genetic material into human chromosomes, a critical step in the pathogenesis of HIV. Raltegravir potassium salt is marketed under the trade name ISENTRESS™ by Merck & Co.
The processes for preparing Raltegravir that are known in the art either require a protection step for the 5-hydroxy group prior to the methylation step, or lead to an impurity resulting from the methylation of the 5-hydroxy group.
U.S. Patent No. 7, 169,780 discloses Raltegravir and preparation thereof, as described in the following reaction scheme:
Scheme 1
J. Med. Chem. 2008, 51 , 5843-5855 discloses another process for preparing Raltegravir as described in the following reaction scheme:
RLT K-salt
Scheme 2 U.S. Publication No. US 2006/0122205 describes an alternative process for preparing Raltegravir, in which the alkylation step does not include a step for protecting the 5-hydroxy group. The process is described in the following reaction scheme:
Scheme 3
Provided herein is an industrially applicable process for preparing RLT-7', RLT-8, RLT-9 and RLT-9-OP, intermediates in the synthesis of Raltegravir, as well as processes for preparing Raltegravir and crystalline forms thereof.
US Publication No. US 2006/0122205, WO 2010/1401 56 and WO 201 1 /
024192 describe the potassium salt of Raltegravir, including amorphous and crystalline forms I, II, III and H I , as well as amorphous and crystalline forms of Raltegravir free- hydroxy. PCT publication No. WO 201 1/123754 describes certain Raltegravir salts and polymorphs, including form V of Raltegravir potassium.
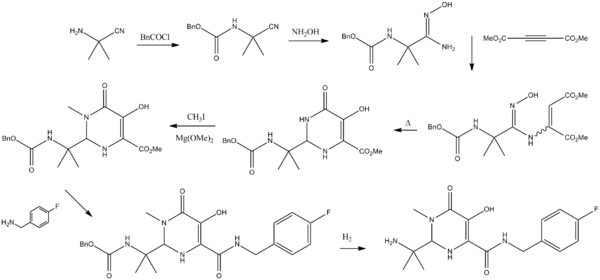
| Conditions:- i. Benzylchloroformate, N,N-diisopropylethylamine, Methyl tert-butyl ether, 20 – 25 °C, 16 h, ii. Hydroxyl amine, Water, 60 °C, 3 h, iii. Dimethyl acetylenedicarboxylate, methanol, Room temperature 2 h then Xylene 90 °C, 2 h, iv. Magnesium methoxide, dimethyl sulfoxide, Methyl iodide, 20 - 25 °C, 2 h, v. 4-fluorobenzyl amine, ethanol, 72 °C, 2 h, vi. 5% Pd/C, methanol, Molybdate sulfuric acid, Hydrogen gas, 50 °C, 3 h, vii. 5-methyl-1,3,4-oxadiazole-2-carbonylchloride, N-methylmorpholine, Tetrahydrofuran, 0 – 5 °C, 2 h |
| preparation of Raltegravir is described in US patent 2006122205A1 and also in WO2006060730. Accordingly, 2-amino-2-methyl-propanenitrile 1 was reacted with benzylchloroformate in presence of N,N-diisopropylethylamine using methyl tert-butyl ether as solvent at ambient temperature to give benzyl N-(1-cyano-1-methyl-ethyl)carbamate 2. Treatment of 2 with hydroxyl amine using water as solvent at elevated temperature give benzyl N-[(2Z)-2-amino-2-hydroxyimino-1,1-dimethyl-ethyl]carbamate 3. The compound 3 was further cyclized with dimethyl acetylenedicarboxylate using methanol as solvent at higher temperature to give methyl 2-(1-benzyloxycarbonylamino-1-methyl-ethyl)-5-hydroxy-6-oxo-1H-pyrimidine-4-carboxylate 4. Compound 4 was then methylated with methyl iodide in presence of magnesium methoxide as base and dimethyl sulfoxide as solvent at ambient temperature to give methyl 2-(1-benzyloxycarbonylamino-1-methyl-ethyl)-5-hydroxy-1-methyl-6-oxo-pyrimidine-4-carboxylate 5. Compound 5 on condensing with 4-fluorobenzyl amine using ethanol as solvent result in to benzyl N-[1-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxo-pyrimidin-2-yl]-1-methyl-ethyl]carbamate 6, which underwent benzyloxy-decarboxylation on hydrogenating with hydrogen gas in presence of 5% Palladium on carbon catalyst and molybdate sulfuric acid using methanol as solvent to give 2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-6-oxo-pyrimidine-4-carboxamide 7. The final step involves condensation of 7 with 5-methyl-1,3,4-oxadiazole-2-carbonylchloride in presence of N-methylmorpholine as base using tetrahydrofuran as solvent at slightly lower temperature to afford N-[1-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxo-pyrimidin-2-yl]-1-methyl-ethyl]-5-methyl-1,3,4-oxadiazole-2-carboxamide also called Raltegravir 8. |

The formation of the hydroxypyrimidone core (3.22) of raltegravir deserves further discussion as its unexpected mechanism was only recently fully elucidated in a joint effort between Merck process chemists and the Houk group at UCLA [91]. These studies combined B3LYP density functional theory with labelling studies and revealed that the most likely pathway involves the formation of a tightly bound polar radical pair 3.31 resulting from thermal homolysis of the N–O bond (Scheme 35). This species subsequently recombines under formation of a C–N bond and a C=O double bond (3.32) allowing for the final cyclocondensation to occur with liberation of methanol. Furthermore these studies were able to disprove a potential alternative [3,3]-sigmatropic rearrangement step by incorporating 15N enriched precursors leading to the formation of pyrimidone 3.22, which is only consistent with a formal [1,3]-sigmatropic rearrangement. Subsequent calculations demonstrated the high energy barrier for such a concerted [1,3]-shift, ultimately leading to the finding of the before-mentioned polar radical pair pathway which is about 8 kcal/mol lower in energy. This is consistent with the experimentally observed rate acceleration in case of the Z-isomer of 3.33 over the E-isomer which was also confirmed by calculations showing an energy gap of 3 kcal/mol.
An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles
Department of Chemistry, University of Durham, South Road, Durham, DH1 3LE, UK
 Corresponding author email
Corresponding author email
Associate Editor: P. R. Hanson
Beilstein J. Org. Chem. 2013, 9, 2265–2319.
check beilstein journals as per link above ...............
this publication allows free usage of data if given proper ref.......................
any objections email me amcrasto@gmail.com or cal +91 9323115463
....................
nmr
Imp roved synthesis of raltegravir
GUO D i2liang et al
Department ofM edicinal Chem istry, China PharmaceuticalUniversity, N anjing 210009;
Journal of China Pharmaceutical University 2009, 40 (4) : 297 - 301

1H NMR (CD3OD) δ: 7.40 (m, 2H) , 7.04 (m , 2H) ,
4.56 (s, 2H ) , 3.46 ( s, 3H ) , 2.65 (s, 3H ) , 1.83 (s,
6H);
13C NMR (CD3OD ) δ: 168.4, 164.8, 163.2,
162.0, 161.9, 160.1, 155.3, 145.8, 136.0, 134.9,
131.0, 116.7, 116.6, 60.2, 43.8, 41.3, 34.8, 27.6,
11.4;
ESI2MS m /z 443 (M )-; LR2MS (EI) m /z 444(M )+; HR2MS ( E I) m /z C20 H21 FN6O5(M )+
calcd444, 155, 7, found 444, 154, 2
second set
lH NMR (399.87 MHz5 CDCI3) δ 12.04 (s, IH), 8.45 (s, IH), 7.94 (t, J = 6.2 Hz, IH), 7.41-736 (m, 2H), 7.08-7.02 (m, 2H)5 4.61 (d, J - 6.2 Hz, 2H), 3.68 (s, 3H), 2.63 (s, 3H), 1.87 (s, 6H).
13C NMR (100.55 MHz, CDCI3) δ 168.3, 166.7, 162.6 (d, JCF=245.7 Hz), 159.6, 159.1, 152.O5 150.4, 147.2, 133.4 (d, JCP=3.2 Hz)5 129.9 (d, JcF=8.0 Hz), 124.1, 115.9 (d, JcF=21.7 Hz), 58.0, 42.7, 33.5, 26.7, 11.4.
.........................
IR
absorption bandsKBR (cm"1) at 832, 1017, 1248, 1350, 1510, 1682, 2995, and 3374
...................
K SALT
Org. Process Res. Dev., 2011, 15 (1), pp 73–83
DOI: 10.1021/op100257r
mp 274.2−275.2 °C. 1H NMR (500 MHz, DMSO-d6) δ: 11.65 (t, J = 6.0 Hz, 1 H), 9.75 (s, 1 H), 7.36 (dd, J = 8.6, 5.7 Hz, 2 H), 7.14 (app. t, J = 8.6 Hz, 2 H), 4.48 (d, J = 6.0 Hz, 2 H), 3.43 (s, 3 H), 2.58 (s, 3 H), 1.73 (s, 6 H);
13C NMR (125 MHz, DMSO-d6) δ: 168.7, 167.0, 166.6, 162.1 (d, JCF = 243 Hz), 159.7, 158.3, 153.1, 139.6, 138.0 (d, JCF = 3 Hz), 130.2 (d, JCF = 8 Hz), 123.7, 116.0 (d, JCF = 22), 58.4, 42.1, 33.3, 28.1 (2 C), 11.7.
.................................
impurities
Org. Process Res. Dev., 2012, 16 (8), pp 1422–1429
DOI: 10.1021/op300077m

.....................
intermediates

N-[(1Z)-1 -amino-1 -(hydroxyimino)-2-memylpropan-2-yl]-5-methyl-l ,3 ,4- oxadiazole-2-carboxamide (IVa) (198 gms) was suspended in methanol (1188 ml) and cooled to 15 to 25°C. Dimethyl acetyl enedicarboxylate (DMAD; 152.8 gms) was added and the reaction mass was stirred for 2 to 3 hours at 25°C. The reaction mass was concentrated under reduced pressure and xylene was added and stirred between 135°C and 125°C for 6 hour. After completion of reaction, the mixture was cooled to 60°C and methanol (170 ml) & methyl tert-butyl ether (MTBE) were added to the reaction mass and stirred for 1 hour. The resultant slurry was filtered and washed with a 9:1 mixture of methanol & methyl tert-butyl ether (MTBE) and dried to give methyl 2-(2-(5-methyl-l ,3,4-oxadiazole-2-carboxamido)propan-2-yl)-l ,6-dihydro-5- hydroxy-6-oxopyrimidine-4-carboxylate (V a).
Yield: 198 gms (66 %).
1H NMR (400 MHz, DMSO d6): δ 12.74 (s, 1H), 10.35 (s, 1H), 9.12 (s, 1 H), 3.81 (s, 3H), 2.58 (s, 3H), 1.59 (s, 6 H);
13C NMR (100 MHz, DMSO d6): δ 166.60, 166.15, 160.19, 159.23, 153.26, 152.87, 145.65, 128.30, 56.60, 52.91 , 26.26, 11.34;
retroviral drugs

elvitegravir
Å for chemical synthesis from carboxylic acids elvitegravir 1 starts, the NIS transformed into acid chloride iodide, 2 , and with 3 condensation 4 . 4 and amino alcohols 5 addition-elimination reaction occurs 6 , 6 in alkaline conditions Shimonoseki ring hydroxyl group protected with TBS after seven , seven and zinc reagent 8 occurred Negishi coupling get nine , the last ninehydrolysis and methoxylated get angstrom for elvitegravir.






Elvitegravir (EVG, JTK-303/GS-9137) is an HIV integrase inhibitor for HIV-1 IIIB, HIV-2 EHO and HIV-2 ROD with IC50 of 0.7 nM, 2.8 nM and 1.4 nM, respectively. Elvitegravir
ReplyDeleteRaltegravir is used along with other medications to treat human immunodeficiency virus (HIV) infection. Raltegravir is in a class of medications called HIV integrase inhibitors. It works by decreasing the amount of HIV in the blood. Buy Raltegravir from onlinegenericmedicine.com (Online Pharmacy), they provide all types of generic medicine, best in class products and best deals. Click here to read more about this medicine.
ReplyDelete