
Ospemifene
CAS Number: 128607-22-7
CAS Number: 128607-22-7
OSPHENA is indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause
Also known as:
- CCRIS 9205
- Deamino-hydroxytoremifene
- Fc-1271
- FC-1271a
- Ospemifene
- Osphena
- UNII-B0P231ILBK
Molecular Formula: C24H23ClO2
Molecular Weight: 378.89 g.mol-1
Molecular Weight: 378.89 g.mol-1
Ospemifene, FC-1271a
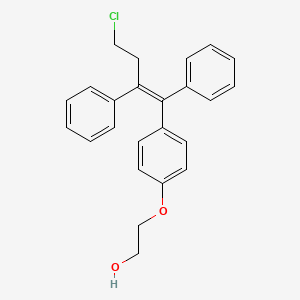
2-[4-[4-Chloro-1,2-diphenyl-1(Z)-butenyl]phenoxy]ethanol
Bone Diseases, Treatment of, ENDOCRINE DRUGS, Gynecological Disorders, Treatment of , Hormone Replacement Therapy, METABOLIC DRUGS, Treatment of Osteoporosis, Treatment of Postmenopausal Syndrome , Selective Estrogen Receptor Modulators (SERM)
..............................
 OSPEMIFINE
OSPEMIFINE
Article 27 March 2013
Shionogi Limited, the London-based European subsidiary of Shionogi & Co., Ltd announced today that on 26th March 2013 the European Medicines Agency (EMA) accepted its Marketing Authorisation Application (MAA) submission for ospemifene for the treatment of vulvar and vaginal atrophy (VVA) in post-menopausal women.
this is already approved by FDA
“We are pleased to announce the MAA submission for ospemifene to the EMA following the US Food and Drug Administration (FDA) approval last month. The acceptance of the MAA submission for ospemifene not only represents an important step forward in expanding the treatment options for women living in Europe with this condition, but it is also an important milestone for Shionogi as it continues to build its business in Europe” said Takashi Takenoshita, CEO of Shionogi Limited.
Osphena (ospemifene) to treat women experiencing moderate to severe dyspareunia (pain during sexual intercourse), a symptom of vulvar and vaginal atrophy due to menopause.
Dyspareunia is a condition associated with declining levels of estrogen hormones during menopause. Less estrogen can make vaginal tissues thinner, drier and more fragile, resulting in pain during sexual intercourse.
Osphena, a pill taken with food once daily, acts like estrogen on vaginal tissues to make them thicker and less fragile, resulting in a reduction in the amount of pain women experience with sexual intercourse.
“Dyspareunia is among the problems most frequently reported by postmenopausal women,” said Victoria Kusiak, M.D., deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research. “Osphena provides an additional treatment option for women seeking relief.”
Osphena’s safety and effectiveness were established in three clinical studies of 1,889 postmenopausal women with symptoms of vulvar and vaginal atrophy. Women were randomly assigned to receive Osphena or a placebo. After 12 weeks of treatment, results from the first two trials showed a statistically significant improvement of dyspareunia in Osphena-treated women compared with women receiving placebo. Results from the third study support Osphena’s long-term safety in treating dyspareunia.
Common side effects reported during clinical trials included hot flush/flashes, vaginal discharge, muscle spasms, genital discharge and excessive sweating.
Osphena is marketed by Florham Park, N.J.-based Shionogi, Inc.
- Shionogi Files a New Drug Application for Ospemifene Oral Tablets 60mg for the Treatment of Vulvar and Vaginal Atrophy – May 9, 2012
- END OF ARTICLE
......................................
Ospemifene (commercial name Osphena produced by Shionogi) is an oral medication indicated for the treatment of dyspareunia - pain during sexual intercourse - encountered by some women, more often in those who are post-menopausal. Ospemifene is aselective estrogen receptor modulator (SERM)[1] acting similarly to an estrogen on thevaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulval and vaginal atrophy."[2]
Ospemifene is used to treat dyspareunia. It is available as a 60 mg tablet that is taken by mouth once a day. The fact that ospemifene can be taken orally is an advantage over other products that are used to treat dyspareunia, because these are generally in a topical dosage form and have to be applied locally.[2] The oral dosage form is much easier and more convenient for patients to administer.
It is "an agonist/antagonist that makes vaginal tissue thicker and less fragile resulting in a reduction in the amount of pain women experience with sexual intercourse."[2] This drug should be used for the shortest amount of time possible due to associated adverse effects.[2]
Approval process
Hormos Medical Ltd., which is a part of QuatRx Pharmaceuticals, filed a patent on January 19, 2005 for a solid dosage form of ospemifene.[5] In March of 2010, QuatRX Pharmaceuticals licensed ospemifene to Shionogi & Co., Ltd. for them to develop it into a product and put it on the market.[6] A New Drug Application (NDA) was submitted to the FDA on April 26, 2012.[7] Amendments to the NDA were submitted in June, July, August, October, and November 2012, and January and February of 2013.[7] It was ultimately approved by the FDA on February 26, 2013.[6]
Preclinial and clinical trials
Preclinical trials were performed in ovariectomized rats to model menopause.[8] Oral ospemifene was compared with raloxifene (another SERM), its metabolites 4-hydroxy ospemifene and 4'-hydroxy ospemifene, estradiol, and ospemifene administered as an intravaginal suppository.[8] Estradiol was used as a positive control and raloxifene was used because it is in the same drug class as ospemifene.[8]Multiple doses of oral ospemifene were tested.[8] 10 mg/kg/day of Ospemifene was found to cause a greater increase in vaginal weight and vaginal epithelial height than 10 mg/kg/day of raloxifene.[8] Vaginal weight had a 1.46x increase after a two week treatment of 10mg/kg/day of ospemifene.[8] The number of progesterone receptors was increased in the vaginal stroma and epithelium, which indicates that ospemifene has "estrogenic activity."[8]
A binding assay was also performed to measure the affinity of ospemifene for the estrogen receptor (ERα and ERβ).[8] The study showed that ospemifene bound ERα and ERβ with similar affinity.[8] Ospemifene bound the estrogen receptors with a lower affinity than estradiol.[8] Ospemifene was shown to be an antagonist of "ERE-mediated transactivation on MCF-7 cells," which the authors concluded indicates "anti-estrogenic activity in breast cancer cells."[8]
Two 12 week phase 3 clinical trials were performed for ospemifene.[9] To evaluate the efficacy of the drug, 4 signs and symptoms of dyspareunia were measured. These included the "change in percent parabasal cells," "change in percent superficial cells," "change in vaginal pH," and "change in most bothersome symptom (vaginal dryness and vaginal pain associated with sexual activity."[9]Ospemifene was more effective than placebo in all four of these categories.[9] A dose-response was also seen in the trial; ospemifene 60 mg had greater efficacy than ospemifene 30 mg.[9] Safety was also evaluated in these phase 3 trials. There was a 5.2% increase in the incidence of hot flushes, 1.6% increase in urinary tract infections, and 0.5% increase in the incidence of headache with ospemifene over placebo.[9] One of the phase 3 trials was double-blinded and randomized and involved 826 women who were post-menopausal.[10]The women in the study were required to have one or more vulvovaginal atrophy (VVA) symptom that was moderate or severe in nature, no more than 5% of cells that were superficial when given a vaginal smear, and have a vaginal pH of at least 5.0.[10] Another phase 3 trial involved 605 women who were between the ages of 40 and 80, were diagnosed with VVA, and whose worst symptom was dyspareunia.[11]
 OSPEMIFENE
OSPEMIFENE
In the first half of the 2013 fiscal year, Osphena® generated 0.1 B yen in revenue, which is probably roughly equivalent to $974, 944 U.S. dollars.[12] When Osphena® was put onto the market, it was predicted to earn $495 million in 2017.[13]
OSPHENA is an estrogen agonist/antagonist. The chemical structure of ospemifene is shown in Figure 1.
Figure 1: Chemical structure
 |
The chemical designation is Z-2-[4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy]ethanol, and has the empirical formula C24H23ClO2, which corresponds to a molecular weight of 378.9. Ospemifene is a white to off-white crystalline powder that is insoluble in water and soluble in ethanol.
Each OSPHENA tablet contains 60 mg of ospemifene. Inactive ingredients include colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate, titanium dioxide, and triacetin.
References
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O (2003). "Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial". Menopause 10 (5): 433–9.doi:10.1097/01.GME.0000063609.62485.27.PMID 14501605.
- Tanzi MG (April 2013). "Ospemifene: New treatment for postmenopausal women.". Pharmacy Today. American Pharmacists Association.
- "FDA approves Osphena for postmenopausal women experiencing pain during sex". FDA News Release (U.S. Food and Drug Administration). 2013-02-26.
- "Ospemifene: Indications, Side Effects, Warnings". Drugs.com.
- EP application 2286806, Lehtola V-M, Halonen K, "Solid formulations of ospemifene", published 2011-02-23, assigned to Hormos Medical Ltd.
- "Shionogi Files a New Drug Application for Ospemifene Oral Tablets 60mg for the Treatment of Vulvar and Vaginal Atrophy". Drugs.com.
- Kusiak V (2013-02-13). "NDA Approval" (PDF). U.S. Food and Drug Administration.
- Unkila M, Kari S, Yatkin E, Lammintausta R (November 2013). "Vaginal effects of ospemifene in the ovariectomized rat preclinical model of menopause". J. Steroid Biochem. Mol. Biol. 138: 107–15.doi:10.1016/j.jsbmb.2013.04.004. PMID 23665515.
- Center for Drug Evaluation and Research (2013-02-26). "Clinical Pharmacology and Biopharmaceutics Review Application Number 203505Orig1s000" (PDF). Office of Clinical Pharmacology Review. U.S. Food and Drug Administration.
- Bachmann GA, Komi JO (2010). "Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study". Menopause 17 (3): 480–6.doi:10.1097/gme.0b013e3181c1ac01. PMID 20032798.
- Portman DJ, Bachmann GA, Simon JA (June 2013). "Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy". Menopause 20 (6): 623–30.doi:10.1097/gme.0b013e318279ba64. PMID 23361170.
- http://www.shionogi.co.jp/en/ir/pdf/e_p131101.pdf. First Half of Fiscal 2013 Financial Results. Nov. 1, 2013.
- http://www.thepharmaletter.com/article/fda-approves-shionogi-s-osphena-for-postmenopausal-women-experiencing-pain-during-sex. ThePharmaLetter
PATENTS
8-8-2012
|
Method for enhancing the bioavailablity of ospemifene
| |
1-21-2011
|
METHOD FOR THE PREPARATION OF THERAPEUTICALLY VALUABLE TRIPHENYLBUTENE DERIVATIVES
| |
11-5-2010
|
METHODS FOR THE INHIBITION OF ATROPHY OR FOR TREATMENT OR PREVENTION OF ATROPHY-RELATED SYMPTOMS IN WOMEN
| |
10-13-2010
|
METHOD FOR THE PREPARATION OF THERAPEUTICALLY VALUABLE TRIPHENYLBUTENE DERIVATIVES
| |
3-18-2009
|
METHODS FOR THE PREPARATION OF FISPEMIFENE FROM OSPEMIFENE
| |
5-11-2007
|
Novel oral formulations of ospemifene
| |
5-11-2007
|
Formulations of fispemifene
| |
1-11-2006
|
Methods for the inhibition of atrophy or for treatment or prevention of atrophy-related symptoms in women
| |
12-9-2005
|
Methods for the inhibition of atrophy or for treatment or prevention of atrophy-related symptoms in women
| |
8-26-2005
|
Solid formulations of ospemifene
|
8-26-2005
|
Method for treatment or prevention of osteoporosis in individuals with high bone turnover
| |
4-6-2005
|
Triphenylalkene derivatives and their use as selective estrogen receptor modulators
| |
6-11-2003
|
Triphenylalkene derivatives and their use as selective estrogen receptor modulators
| |
6-13-2001
|
Method for the treatment of vaginal dryness and sexual dysfunction in women during or after the menopause
|
"SERM"s (selective estrogen receptor modulators) have both estrogen-like and antiestrogenic properties (Kauffman & Bryant, 1995). The effects may be tissue-specific as in the case of tamoxifen and toremifene which have estrogen-like effects in the bone, partial estrogen-like effect in the uterus and liver, and pure antiestrogenic effect in breast cancer.
Raloxifene and droloxifen are similar to tamoxifen and toremifene, except that their antiestrogenic properties dominate. Based on the published information, many SERMs are more likely to cause menopausal symptoms than to prevent them. They have, however, other important benefits in elderly women: they decrease total and LDL cholesterol, thus deminishing the risk of cardiovascular diseases, and they may prevent osteoporosis and inhibit breast cancer growth in postmenopausal women.
Ospemifene is the Z-isomer of the compound of formula (I)
and it is one of the main metabolites of toremifene, is known to be an estrogen agonist and antagonist (Kangas, 1990; International patent publications WO 96/07402 and WO 97/32574 ). The compound is also called (deaminohydroxy)toremifene and it is also known under the code FC-1271a. Ospemifene has relatively weak estrogenic and antiestrogenic effects in the classical hormonal tests (Kangas, 1990). It has anti-osteoporosis actions and it decreases total and LDL cholesterol levels in both experimental models and in human volunteers (International patent publications WO 96/07402 and WO 97/32574 ). It also has antitumor activity in an early stage of breast cancer development in an animal breast cancer model. Ospemifene is also the first SERM which has been shown to have beneficial effects in climacteric syndromes in healthy women. The use of ospemifene for the treatment of certain climacteric disorders in postmenopausal women, namely vaginal dryness and sexual dysfunction, is disclosed in WO 02/07718 . The published patent application WO 03/103649 describes the use of ospemifene for inhibition of atrophy and for the treatment or prevention of atrophyrelated diseases or disorders in women, especially in women during or after the menopause.
SYNTHESIS

credit chemdrug
The condensation of desoxybenzoin (I) with 2-(benzyloxy)ethyl bromide (II) by means of aqueous 48% NaOH containing triethylbenzylammonium chloride (TEBAC) gives 4-(benzyloxy)-1,2-diphenyl-1-butanone (III), which by reaction with the Grignard reagent (IV) – prepared from 4-(tetrahydropyranyloxy)phenyl bromide (V) and Mg in THF – yields the triphenylbutanol derivative (VI). Elimination of the THP-protecting group of compound (VI) by means of H2SO4 in ethanol/water at room temperature affords the triphenylbutanol derivative (VII), which is debenzylated by hydrogenation with H2 over Pd/C in ethanol to provide the butane-1,4-diol derivative (VIII). Cyclization of the butane-1,4-diol (VIII) by means of H2SO4 in hot ethanol/water gives 2-(4-hydroxyphenyl)-2,3-diphenyltetrahydrofuran (IX), which is treated with 48% HBr in refluxing AcOH to yield a mixture of (E)- and (Z)-4-(4-hydroxyphenyl)-3,4-diphenyl-3-buten-1-ol (X), which is separated by chemical work up. The phenolic OH group of the desired (Z)-isomer (X) is condensed with 2-(benzyloxy)ethyl bromide (II) by means of NaOH and tetrabutylammonium bromide in refluxing toluene/ water to afford the benzyloxyethyl ether (XII). Reaction of the aliphatic OH group of ether (XII) with PPh3 and CCl4 in acetonitrile provides the corresponding chloro derivative (XIII), which is finally debenzylated with H2 over Pd/C in ethyl acetate/ethanol.

Sorbera, L.A.; Castar, J.; Bay
Ospemifene. Drugs Fut 2004, 29, 1, 38
........................................................
US 4996225; US 5491173,
WO 9732574, WO 9607402,

The condensation of desoxybenzoin (I) with tetrahydropyranyl ether (II) in aq. 48% NaOH containing TEBAC gives 1,2-diphenyl-4-(tetrahydropyranyloxy)-1-butanone (III), which by a Grignard condensation with 4-methoxyphenylmagnesium bromide (IV) in THF yields the monoprotected triphenylbutanediol (V). The deprotection of (V) with H2SO4 in ethanol/water at room temperature affords the triphenylbutane-1,4-diol (VI), which is cyclized with H2SO4 in hot ethanol/water to provide 2-(4-methoxyphenyl)-2,3-diphenyltetrahydrofuran (VII). The reaction of (VII) with 48% HBr in refluxing acetic acid gives a mixture of (E)- and (Z)-4-(4-hydroxyphenyl)-3,4-diphenyl-1-butanol that is separated by chemical working up to obtain the desired (Z)-isomer (VIII). The condensation of the phenolic OH of (VIII) with benzyl protected 2-bromoethanol (IX) by means of NaOH and tetrabutylammonium bromide in refluxing toluene/water gives the benzyloxyethyl ether (X). The reaction of the aliphatic OH group of (X) with PPh3 and CCl4 in acetonitrile yields the corresponding chloro derivative (XI), which is finally debenzylated by hydrogenation with H2 over Pd/C in ethyl acetate/ethanol.

.....................................................................
SYNTHESIS
Ospemifene simple structure, its point is to control the synthesis of the product cis-trans isomerization of the double bond. Chloride 1 and benzene ( 2 ) occurs pay - acylation reaction 3 . Ester4 aluminum trichloride under the action of Fries rearrangement of 5 , 5 on the propylene oxide under alkaline conditions to obtain 6 , 6 and 3 McMurry coupling occurs directly generated Ospemifene.
...................................................
SYNTHESIS
- The common starting material in the syntheses of (Ib), namely compound (II), is previously known (Toivola, 1990; EP 0095875 ). According to a method disclosed in EP 095875 , this compound was prepared by dealkylation of a corresponding ether to give (II). The method may be used to produce a mixture of isomers of compounds (Ib), but most preferably is used to prepare the pure E- and Z-isomers of this compound.
- Particularly in case the Z-isomer of the compound (Ib) is desired, a preferable method for the synthesis of compound (II) is a McMurry reaction of commercially available starting materials, 4-hydroxybenzophenone with 3-chloropropiophenone. The McMurry reaction is a well-known reductive coupling of ketones involving two steps: (1) a single electron transfer to the carbonyl groups from an alkali metal, followed by (2) deoxygenation of the 1,2-diol with low-valent titanium to yield the alkene. This reaction produces mainly the Z-isomer of compound (II)

- The alkylation in step a) is carried out in an organic solvent, preferably carried out in tetrahydrofuran. It is also preferable to add a base to the solvent, most preferably sodium hydride
- Zinc (15.0 g, 0.23 mol) and tetrahydrofuran (THF) (180 ml) was added to the reaction vessel and cooled to -10 °C. Titan tetrachloride was added dropwise to the mixture (21.6 g, 0.114 mol) at about -10 °C. After the addition was completed the mixture was refluxed for two hours. Then the mixture was cooled to 40 °C and 4-hydroxybenzophenone (7.68 g, 0.039 mol) and 3-chloropropiophenone (6.48 g, 0.039 mol) dissolved in THF (75 ml) were added to the mixture. Refluxing was continued for additional 3.5 hours. The cooled reaction mixture was poured in aqueous potassium carbonate solution (21 g K2CO3 + 210 ml water) and allowed to stand overnight at the ambient temperature. The mixture was filtered and the precipitate was washed with THF. The filtrate was evaporated to dryness. The residue was dissolved in ethyl acetate and washed with water. Ethyl acetate phase was evaporated to dryness and the residue was crystallized first from methanol-water (8:2) and then from methanol-water (9:1). Yield 5.4 g.
- Z-isomer: 1H NMR (CDCl3): 2.92 (t, 2H, =CH 2CH2Cl), 3.42 (t, 2H, =CH2 CH 2 Cl), 6.48 (d, 2H, aromatic proton ortho to hydroxy), 6.75 (d, 2H, aromatic proton meta to hydroxy), 7.1-7.4 (m, 10H, aromatic protons)
- EXAMPLE 14-(4-Chloro-1,2-diphenyl-but-1-enyl)phenol (Compound II)
EXAMPLE 2
2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethanol (Compound Ib)
- 4-(4-Chloro-1,2-diphenyl-but-1-enyl)phenol (0.23 g, 0.689 mmol) was dissolved in tetrahydrofuran (3 ml) under nitrogen atmosphere. Sodium hydride (0.025 g, 1.03 mmol) was added to the solution and the mixture was stirred at room temperature for an hour. 2-(2-iodo-ethoxy)-tetrahydropyran (0.3 g, 1.17 mmol) was added and the mixture was refluxed for 2 hours. Additional portions of 2-(2-iodo-ethoxy)-tetrahydro-pyran (0.5 g, 2 mmol) were added to the mixture during seven hours. After cooling and adding water, THF was evaporated and the mixture was extracted three times with ethyl acetate. The organic phase was washed with 2 N aqueous sodium hydroxide and water, dried with sodium sulphate and evaporated to dryness. The residue (which is Compound (IV) where Pr is tetrahydropyranyl) was dissolved in ethanol and acidified with 2 N aqueous hydrogen chloride solution. The mixture was stirred at room temperature over night, evaporated and extracted with dichloromethane. After washing with water the organic phase was dried (Na2SO4) and evaporated. The residue was purified by flash chromatography with dichloromethane/methanol 9.5/0.5 as eluent. Yield 0.17 g, 59 %.
- Z-isomer, 1H NMR (CDCl3): 2.92 (t, 2H, =CH 2CH2Cl), 3.42 (t, 2H, =CH2 CH 2 Cl), 3.85-3.89 (m, 4H, OCH 2 CH 2), 6.56 (d, 2H, aromatic proton ortho to hydroxy), 6.80 (d, 2H, aromatic proton meta to hydroxy), 7.1-7.43 (m, 10H, aromatic protons).
EXAMPLE 3
2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethanol (Compound Ib)
- The compound was prepared by the same method as described in Example 2 using 2-(2-iodo-ethoxymethyl)-benzene as a reagent and removing the benzylic protecting group using the method described in Example (e) ofUS Patent No. 6,891,070 B2 . Briefly, the removal is carried out under a nitrogen atmosphere, in the presence of Zn powder and acetyl chloride.
- EXAMPLE 5
2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethanol (Compound Ib)
- [4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-acetic acid ethyl ester (Example 4) was dissolved in tetrahydrofuran at room temperature under nitrogen atmosphere. Lithium aluminium hydride was added to the solution in small portions until the reaction was complete. The reaction was quenched by adding saturated ammonium chloride solution to the mixture. The product was extracted into toluene, which was dried and evaporated in vacuo. The yield 100 mg, 43 %.
- 1H NMR (CDCl3): 2.92 (t, 2H, =CH 2CH2Cl), 3.42 (t, 2H, =CH2 CH 2 Cl), 3.85-3.89 (m, 4H, OCH 2 CH 2), 6.56 (d, 2H, aromatic proton ortho to hydroxy), 6.80 (d, 2H, aromatic proton meta to hydroxy), 7.1-7.43 (m, 10H, aromatic protons).
................................
e) 2-{2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)phenoxy]ethoxy}ethanol:
Z-1-{4-[2-(2-Benzyloxy-ethoxy)ethoxy]phenyl}-4-chloro-1,2-diphenyl-but-1-ene (3.8 g, 7.4 mmol) is dissolved in ethyl acetate under nitrogen atmosphere, Zn powder (0.12 g, 1.85 mmol) and acetyl chloride (1.27 g, 16.3 mmol) are added and the mixture is stirred at 50° C. for 3 h (Bhar, 1995). The reaction mixture is cooled to room temperature, water (10 ml) is added and stirring is continued for additional 10 min. The aqueous layer is separated and the organic phase is washed with 1 M aqueous hydrogen chloride solution and with water. Ethyl acetate is evaporated and the residue is dissolved in methanol (16 ml) and water (4 ml). The acetate ester of the product is hydrolysed by making the mixture alkaline with sodium hydroxide (1 g) and stirring the mixture at room temperature for 1 h. Methanol is evaporated, water is added and the residue is extracted in ethyl acetate and washed with 1 M hydrogen chloride solution and with water. Ethyl acetate is evaporated and the residue is dissolved in toluene (25 ml), silica gel (0.25 g) is added and mixture is stirred for 15 min. Toluene is filtered and evaporated to dryness.
The residue is crystallised from heptane-ethyl acetate (2:1). The yield is 71%.
Z-isomer: 1H NMR (CDCl3): 2.92 (t, 2H), 3.41 (t, 2H), 3.58-3.63 (m, 2H), 3.69-3.80 (m, 4H), 3.96-4.01 (m, 2H), 6.56 (d, 2H), 6.78 (d, 2H), 7.10-7.40 (m, 10H).
E-2-{2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)phenoxy]ethoxy}ethanol is prepared analogously starting from E-1-{4-[2-(2-benzyloxy-ethoxy)ethoxy]phenyl}-4-chloro-1,2-diphenyl-but-1-ene. The product is purified by flash chromatography with toluene-methanol (10:0.5) as eluent.
E-isomer: 1H NMR (CDCl3): 2.97 (t, 2H), 3.43 (t, 2H), 3.65-3.79 (m, 4H), 3.85-3.90 (m, 2H), 4.13-4.17 (m, 2H), 6.85-7.25 (m, 2H).
Debenzylation of 1-{4-[2-(2-benzyloxy-ethoxy)ethoxy]phenyl}-4-chloro-1,2-diphenyl-but-1-ene is also carried out by hydrogenation with Pd on carbon as a catalyst in ethyl acetate-ethanol solution at room temperature.
..................
PATENT
http://www.google.com/patents/WO2014060639A1?cl=en
EXAMPLE 5. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospemifene) by base hydrolysis of pivaloyl-groiip
; . (Z)-2-(4-(4-Chloro- l ,2-diphenylbut-l-en- l-yl)phenqxy)ethyl pivalate ( 1 g, 2.16 mmol) was dissolved in THF (8 ml) followed by addition of MeOH (1 ml) and water (1 ml). Sodium hydroxide (0.1 g, 2.5 mmol) was added in orie portion and the reaction was stirred at room temperature for 12 h. After completion of the reaction the mixture was partitioned between water (20 ml) and EtOAc (20 ml). Organic phase was washed with water (20 ml) and brine (20 ml); dried (Na2S04), filtered, and concentrated: The residue was crystallized from -PrOH yielding ospernifene (0:29 g, 35 %) as a white solid.
1H-NMR (400 MHz, CDC13) δ (ppm): 7.37 (2H, t, 7=8Hz, ArH), 7.29 (3Η, t, J=7.2Hz, ArH), 7.20 (2Η, t,7=7.6Hz, ArH), 7.16-7.13 (3Η, m, ArH), 6.80 (2Η, d, J=8.8Hz, ArH), 6.57 (2Η, d, 7=8.8Hz, ArH), 3.94 (2Η, t, y=4.4Hz, ArOCH2CH2OH), 3.87 (2H, m, ArOCH2CH OH), 3.42 (2H, t, J=7.2Hz, C1CH2CH2), 2.92 (2H, t, 7=7.2Hz, C1CH2CH2), 1.95 (1Η, t, 7=6.4Hz, OH).
13C- NMR (100 MHz, CDC13) δ (ppm): 157.2, 143.2, 142.1 , 141.3, 2 x 135.7, 132.2, 130.0, 129.8, 128.8, 128.7, 127.4, 127.0, 113.9, 69.3, 61.8, 43.3, 39.0.
EXAMPLE 6. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospernifene) by reductive cleavage of pivaloyl-grou
(Z)-2-(4-(4-Chloro- 1 ,2-diphenylbut- 1 -en- 1 -yl)phenoxy)ethyl pivalate (3.5 g, 7.56 mmol) was dissolved in toluene (35 ml) and stirred under nitrogen for 5 min at room temperature. Lithium aluminium hydride solution (1 M in THE) (7.56 ml, 7.56 n mbi) was added dropwise to the reaction and the mixture was stirred at room temperature for 30 min. After HPLC indicated completion, the reaction was quenched by addition of saturated NH4Cl-sblution (75 ml). Additional amount of toluene (30 ml) was added and the phases were separated. The organic phase was washed with water (50 ml), brine (50 ml), dried (Na2S04), filtered and concentrated in vacuo. The residue was crystallized from 90 % MeOH yielding ospernifene (1 ,75 g, 61 9c) as a white solid.
1H NMR PREDICT
13C NMR PREDICT
..................
PATENT
http://www.google.com/patents/WO2014060639A1?cl=en
EXAMPLE 5. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospemifene) by base hydrolysis of pivaloyl-groiip
; . (Z)-2-(4-(4-Chloro- l ,2-diphenylbut-l-en- l-yl)phenqxy)ethyl pivalate ( 1 g, 2.16 mmol) was dissolved in THF (8 ml) followed by addition of MeOH (1 ml) and water (1 ml). Sodium hydroxide (0.1 g, 2.5 mmol) was added in orie portion and the reaction was stirred at room temperature for 12 h. After completion of the reaction the mixture was partitioned between water (20 ml) and EtOAc (20 ml). Organic phase was washed with water (20 ml) and brine (20 ml); dried (Na2S04), filtered, and concentrated: The residue was crystallized from -PrOH yielding ospernifene (0:29 g, 35 %) as a white solid.
1H-NMR (400 MHz, CDC13) δ (ppm): 7.37 (2H, t, 7=8Hz, ArH), 7.29 (3Η, t, J=7.2Hz, ArH), 7.20 (2Η, t,7=7.6Hz, ArH), 7.16-7.13 (3Η, m, ArH), 6.80 (2Η, d, J=8.8Hz, ArH), 6.57 (2Η, d, 7=8.8Hz, ArH), 3.94 (2Η, t, y=4.4Hz, ArOCH2CH2OH), 3.87 (2H, m, ArOCH2CH OH), 3.42 (2H, t, J=7.2Hz, C1CH2CH2), 2.92 (2H, t, 7=7.2Hz, C1CH2CH2), 1.95 (1Η, t, 7=6.4Hz, OH).
13C- NMR (100 MHz, CDC13) δ (ppm): 157.2, 143.2, 142.1 , 141.3, 2 x 135.7, 132.2, 130.0, 129.8, 128.8, 128.7, 127.4, 127.0, 113.9, 69.3, 61.8, 43.3, 39.0.
EXAMPLE 6. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospernifene) by reductive cleavage of pivaloyl-grou
(Z)-2-(4-(4-Chloro- 1 ,2-diphenylbut- 1 -en- 1 -yl)phenoxy)ethyl pivalate (3.5 g, 7.56 mmol) was dissolved in toluene (35 ml) and stirred under nitrogen for 5 min at room temperature. Lithium aluminium hydride solution (1 M in THE) (7.56 ml, 7.56 n mbi) was added dropwise to the reaction and the mixture was stirred at room temperature for 30 min. After HPLC indicated completion, the reaction was quenched by addition of saturated NH4Cl-sblution (75 ml). Additional amount of toluene (30 ml) was added and the phases were separated. The organic phase was washed with water (50 ml), brine (50 ml), dried (Na2S04), filtered and concentrated in vacuo. The residue was crystallized from 90 % MeOH yielding ospernifene (1 ,75 g, 61 9c) as a white solid.
1H NMR PREDICT
13C NMR PREDICT
//////////






No comments:
Post a Comment