Gold nanoparticles help target, quantify breast cancer gene segments in a living cell | lyranara.me:
'via Blog this'
MEDICINAL CHEMISTRY AT ITS BEST, Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, worlddrugtracker, helping millions with websites, 6 million hits on google, one lakh connections worldwide, email amcrasto@gmail.com, call +91 9323115463 India
Pages
- Home
- ABOUT ME
- FLOZIN SERIES 1/2
- SARTAN SERIES
- DRUG LEAD AND CHEMBASE
- DRUG INFO PORTAL
- Setron series
- PITANT SERIES
- VIR SERIES ..HEP C VIRUS 1/2
- SENTAN SERIES
- List of drugs 2012 FDA
- List Of drugs 2014 FDA
- GLUSTAT SERIES
- COXIB SERIES
- FLOXACIN SERIES
- Brand-Name Drug Patent Expiration Search
- Gravir series
- List of drugs 2013 FDA
- PROST SERIES
- ROLIMUS SERIES
- LIDOMIDE SERIES
Wednesday, 23 April 2014
Saturday, 19 April 2014
Emerging classes of armed antibody therapeutics against cancer
Med. Chem. Commun., 2014, 5,408-431
DOI: 10.1039/C3MD00360D, Review Article
DOI: 10.1039/C3MD00360D, Review Article
Christian Hess, Dario Venetz, Dario Neri
There is a trend in oncology towards the development of 'armed' antibody products. In this article,
There is a trend in oncology towards the development of 'armed' antibody products. In this article,
some of the most advanced preclinical and clinical activities in the field of armed
antibodies are reviewed.
antibodies are reviewed.
Monoclonal antibodies represent the largest and fastest growing type of biopharmaceuticals.
Their commercial and clinical success has fueled research activities aiming to improve safety
and efficacy. In oncology, there is a trend towards the development of ‘armed’ antibody products,
in which the immunoglobulin moiety serves for the selective in vivo pharmacodelivery
of bioactive payloads such as cytotoxic drugs, bispecific antibodies,
radionuclides or cytokines to sites of disease, thereby sparing healthy tissues.
In this article, we review some of the most advanced preclinical and clinical
activities in the field of armed antibodies and present a personal perspective
on the opportunities and challenges associated with the use of this type of anti-cancer therapeutics.
Their commercial and clinical success has fueled research activities aiming to improve safety
and efficacy. In oncology, there is a trend towards the development of ‘armed’ antibody products,
in which the immunoglobulin moiety serves for the selective in vivo pharmacodelivery
of bioactive payloads such as cytotoxic drugs, bispecific antibodies,
radionuclides or cytokines to sites of disease, thereby sparing healthy tissues.
In this article, we review some of the most advanced preclinical and clinical
activities in the field of armed antibodies and present a personal perspective
on the opportunities and challenges associated with the use of this type of anti-cancer therapeutics.
Thursday, 17 April 2014
Wednesday, 16 April 2014
Carbohydrate Derivatives and Glycomimetic Compounds in Established and Investigational Therapies of Type 2 Diabetes Mellitus « New Drug Approvals
Tuesday, 15 April 2014
Saturday, 12 April 2014
Tuesday, 8 April 2014
Ioforminol (GE-145; AN-113111) as an iv contrast agent (Phase 2)
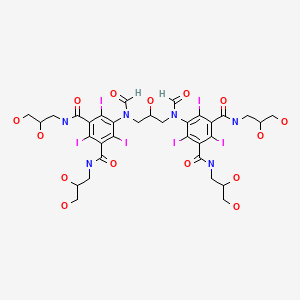
ioforminol
Ioforminol [INN], UNII-95FNF21CDN, 1095110-48-7, FEK-256-062
5-[formyl-[3-[formyl-[3,5-bis(2,3-dihydroxypropylcarbamoyl)-2,4,6- triiodophenyl]amino]-2-hydroxypropyl]amino]-N,N’-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene- 1 ,3-dicarboxamide
1,3-Benzenedicarboxamide, 5,5′-[(2-hydroxy-1,3-
propanediyl)bis(formylimino)]bis[N1,N3-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
1,3-Benzenedicarboxamide, 5,5′-[(2-hydroxy-1,3-
propanediyl)bis(formylimino)]bis[N1,N3-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
All-ambo-5,5'-[2-hydroxypropane-1,3-diylbis(formylazanediyl)]bis[N,N'-bis(2,3-
dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide]
dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide]
MOLECULAR FORMULA C33H40I6N6O15
MOLECULAR WEIGHT 1522.1
SPONSOR GE HealthCare Ltd
CODE DESIGNATION FEK-256-062
CAS REGISTRY NUMBER 1095110-48-7
WHO NUMBER 9245
Visualisation of anatomical structures of the body during computed tomography for diagnostic purposes
All diagnostic imaging is based on the achievement of different signal levels from different structures within the body. Thus, in X-ray imaging for example, for a given body structure to be visible in the image, the X-ray attenuation by that structure must differ from that of the surrounding tissues. The difference in signal between the body structure and its surroundings is frequently termed contrast and much effort has been devoted to means of enhancing contrast in diagnostic imaging since the greater the contrast between a body structure and its surroundings the higher the quality of the images and the greater their value to the physician performing the diagnosis. Moreover, the greater the contrast the smaller the body structures that may be visualized in the imaging procedures, i.e. increased contrast can lead to increased spatial resolution. The diagnostic quality of images is strongly dependent on the inherent noise level in the imaging procedure, and the ratio of the contrast level to the noise level can thus be seen to represent an effective diagnostic quality factor for diagnostic images.
For the last 50 years the field of X-ray contrast agents has been dominated by soluble iodine containing compounds. Commercial available contrast media containing iodinated contrast agents are usually classified as ionic monomers such as diatrizoate (Gastrografen™), ionic dimers such as ioxaglate (Hexabrix™), nonionic monomers such as iohexol (Omnipaque™), iopamidol (Isovue™), iomeprol (lomeron™) and the non-ionic dimer iodixanol (Visipaque™). The most widely used commercial non-ionic X-ray contrast agents such as those mentioned above are considered safe. Contrast media containing iodinated contrast agents are used in more than 20 million of X-ray examinations annually in the USA and the number of adverse reactions is considered acceptable. However, since a contrast enhanced X- ray examination will require up to about 200 ml contrast media administered in a total dose, there is a continuous drive to provide improved contrast media.
Achieving improvement in such a diagnostic quality factor has long been and still remains an important goal.
In techniques such as X-ray, one approach to improve the diagnostic quality factor has been to introduce contrast enhancing materials formulated as contrast media into the body region being imaged. Thus for X-ray, early examples of contrast agents were insoluble inorganic barium salts which enhanced X-ray attenuation in the body zones into which they distributed. For the last 50 years the field of X-ray contrast agents has been dominated by soluble iodine containing compounds.
Commercial available contrast media containing iodinated contrast agents are usually classified as ionic monomers such as diatrizoate (marketed e.g. under the trade mark Gastrografen™), ionic dimers such as ioxaglate (marketed e.g. under the trade mark Hexabrix™), nonionic monomers such as iohexol (marketed e.g. under the trade mark Omnipaque™), iopamidol (marketed e.g. under the trade mark Isovue™), iomeprol (marketed e.g. under the trade mark Iomeron™) and the non-ionic dimer iodixanol (marketed under the trade mark Visipaque™). The clinical safety of iodinated X-ray contrast media has continuously been improved over the recent decades through development of new agents; from ionic monomers (Isopaque™) to non-ionic monomers (e.g. Omnipaque™) and non-ionic dimers (e.g. Visipaque™).
The utility of the contrast media is governed largely by its toxicity, by its diagnostic efficacy, by adverse effects it may have on the subject to which the contrast medium is administered, but also by the ease of production, storage and administration. The toxicity and adverse biological effects of a contrast medium are contributed to by the components of the formulation medium, i.e. of the diagnostic composition, e.g. the solvent or carrier as well as the contrast agent itself and its components such as ions for the ionic contrast agents and also by its metabolites.
The manufacture of non-ionic X-ray contrast media involves the production of the active
pharmaceutical ingredient (API), i.e. the contrast agent prepared in the primary production, followed by the formulation into the drug product, herein denoted the X-ray composition, prepared in the secondary production. In the preparation of an X-ray composition, the contrast agent is admixed with additives, such as salts, optionally after dispersion in a physiologically tolerable carrier. The contrast agent has to be completely solved in the carrier when additives are included and the composition is prepared. A well-known process for preparing X-ray compositions includes heating the contrast agent in the carrier, such as water for injection, to ensure complete dissolution. For instance, for the contrast media Visipaque™ the secondary production process includes dissolution of the contrast agent iodixanol in water for injection and heating to about 98 °C. Heating at this temperature for an adequate period of time ensures that the contrast agent is completely dissolved.
However, different X-ray contrast agents have different solubility. For instance WO 2009/008734 of GE Healthcare AS discloses a new class of compounds and their use as X-ray contrast agents. The compounds are dimers containing two linked iodinated phenyl groups. Compound I, now called
Ioforminol, falling within the formula I of WO2009/008734, has been found by the applicant to have particularly favourable properties. Ioforminol is supersaturated at the relevant storage conditions.
Compound I, Ioforminol:
5-[formyl-[3-[formyl-[3,5-bis(2,3-dihydroxypropylcarbamoyl)-2,4,6- triiodophenyl]amino]-2-hydroxypropyl]amino]-N,N’-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene- 1 ,3-dicarboxamide.
A solution in which the concentration of the solute (API) exceeds the equilibrium solute concentration at a given temperature is said to be supersaturated. This is possible because the solute does not precipitate immediately when the solution is cooled below the saturation temperature. Such solutions are denoted supersaturated.
As the solubility of Ioforminol decreases with decreasing temperature, the supersaturation increases. At room temperature the solubility of Ioforminol is limited. To achieve solutions with a concentration higher than the thermodynamic equilibrium concentration, at room temperature, Ioforminol is dissolved at a temperature above room temperature. When a clear solution has been achieved the solution is cooled and enters a state defined as supersaturated.
Supersaturated solutions are thermodynamically unstable and prone to nucleate and therefore to precipitate on storage. Among several factors, the onset of the precipitation depends on the degree of supersaturation, presence of the crystals of the solute and foreign particles such as dust or other impurities, i.e. purity, and storage temperature of the solution.
The injection solution of Ioforminol, i.e. the administrable X-ray composition, is highly supersaturated. The nucleation (precipitation) in the injection solution at storage conditions is strongly undesirable. The physical stability of the solution, i.e. prevention of the nucleation for a certain time at storage conditions, may be improved substantially by heat treatment of the solution well above its saturation temperature for a sufficiently long period of time.
WO2011/117236 of the applicant is directed to a process involving hea treatment at low pH to avoid degradation and precipitation of an X-ray contrast agent composition. However, a high heat load is needed to obtain a seed- free solution. This heat load causes a greater degradation of the product and a lower pH in the final product resulting in liberation of iodine. This sets a restriction to the total heat load that may be given to the formulated solution.
………………..
X-ray contrast media containing a chemical compound as the active pharmaceutical ingredient(s) having two triiodinated phenyl groups linked by a linking group are usually referred to as dimeric contrast agents or dimers. During the years a wide variety of iodinated dimers have been proposed. Currently, one contrast medium having an iodinated non-ionic dimer as the active pharmaceutical ingredient is on the market^ the product Visipaque™ containing the compound iodixanol. In WO2009/008734 of the applicant a novel dimeric contrast agent named loforminol is disclosed.
The properties of this is described in more detail in the publications Chai et al. “Predicting cardiotoxicity propensity of the novel iodinated contrast medium GE-145: ventricular fibrillation during left coronary arteriography in pigs”, Acta Radiol, 2010, and in Wistrand, L.G., et al “GE-145, a new low-osmolar dimeric radiographic contrast medium”, Acta Radiol, 2010. loforminol (GE-145) is named Compound 1 herein and has the following structure:
Compound 1 :
5,5′-(2-Hydroxypropane-1 ,3-diyl)bis(formylazanediyl)bis(N1 ,N3-bis(2,3- dihydroxypropyl)-2,4,6-triiodoisophthalamide)
The manufacture of non-ionic X-ray contrast media involves the production of the chemical drug, the active pharmaceutical ingredient (API), i.e. the contrast agent, followed by the formulation into the drug product, herein denoted the X-ray composition. WO2009/008734 of the applicant provides a synthetic route for preparing the API loforminol.
loforminol can e.g., as provided by the general preparation description and Example 1 of WO2009/008734, be synthesized from 5- amino-N,N’-bis-(2,3-dihydroxy-propyl)-2,4,6-triiodo-isophthalamide (compound (4)), which is commercially available. The preparation of this compound is known from the synthesis of both iohexol and iodixanol and can also be prepared from 5- nitroisophthalic acid for instance as described in WO2006/016815, including hydrogenation and subsequent iodination e.g. by iodine chloride, I CI. Alternatively,
5-amino-2,4,6-triiodoisophthalic acid may be used, which is commercially available precursor, e.g. from Sigma-Aldrich. The free amino group of the isophthalamide compound (compound (4)) is then acylated and the hydroxyl groups in the substituents may also be protected by acylation. The protecting groups may be removed for example by hydrolysis to give N1 ,N3-bis(2,3-dihydroxypropyl)-5- formylamino-2,4,6-triiodoisophthalamide.
In a dimerization step this is reacted e.g. with epichlorohydrin to provide the loforminol contrast agent compound. The state of the art synthesis of loforminol, as disclosed in examples 1 and 2 of WO2009/008734, is shown in Scheme 1 below.
Scheme 1 .
As described in WO2009/008734 compound 3 is a mixture comprising 1 – formylamino-3,5-bis(2,3-bis(formyloxy)propan-1 -ylcarbamoyl)-2,4,6-trioodobenzene, and X is then a formyl group. In each synthetic step it is important to optimize the yield and minimize the production of impurities. The problem to be solved by the present invention may be regarded as the provision of optimizing the process for preparation of compound mixture (3) of scheme 1 , i.e. a mixture comprising 1 -formylamino-3,5-bis(2,3- bis(formyloxy)propan-1 -ylcarbamoyl)-2,4,6-trioodobenzene.
The process is hence directed to the preparation of compound mixture (3) by the formylation of the amino function of 5-amino-N1 ,N3-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (4), including a work-up procedure.
Examples
Example 1 : Preparation of compound mixture (3) comprising 1-formylamino- 3,5-bis(2,3-bis(formyloxy)propan-1-ylcarbamoyl)-2,4,6-trioodobenzene
5-amino-N1 ,N3-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (compound (4)) (7.5 kg, 10.6 moles) was dissolved in formic acid (4.9 I) and heated to 45 until a clear solution was obtained (~4 hours), then the thick amber solution was cooled to 10 °C.
Formic acid (9.4 I) was charged into a different reactor and cooled to 10 <€, after reaching the target temperature acetic anhydride was added at such a rate that the temperature did not exceeded 15 <€.
After 2.5 hours all acetic anhydride was added to the formic acid and the mixed anhydride solution was added drop wise to the compound (4) solution. The rate of addition was adjusted so that the temperature never exceeded 20 °C. After 2 hours all mixed anhydride had been added and the reaction was left stirring at 15 °C for additional 1 hour. Isopropanol (4.9 I) was added carefully and the suspension became noticeable thicker and was left stirring at ambient temperature. After 16 hours the reaction slurry was filtered on a vacuum nutch and washed with isopropanol (3 * 1 .5 I) to give compound mixture (3) comprising 1 -formylamino-3,5- bis(2,3-bis(formyloxy)propan-1 -ylcarbamoyl)-2,4,6-trioodobenzene as a dense white powder (7.98kg). The quantitative yield with regards to N-formylation was > 99 %.
…………….
Preparation of intermediates (when not commercially available)
The precursors to the compounds of formulas (IVa) and (IVb), the tri-iodinated phenyl groups having a free amino group are commercially available or can be produced following procedures described or referred to e.g. in WO95/35122 and WO98/52911. 5-amino-2,4,6-triiodo-isophtalic acid for example is available e.g. from Aldrich and 5-amino-2,4,6-triiodo-N,N’-bis(2,3-dihydroxypropyl)-isophtalamide is commercially available e.g. from Fuji Chemical Industries, Ltd.
Examples of commercial available precursors of the compounds of formulas (IVa) and (IVb), either commercially available or previously described in the literature include:
5-Amino-N,N’-bis-(2,3-dihydroxy-propyl)-2,4,6-triiodo-isophthalamide
5-Amino-N-(2,3-dihydroxy-propyl)-N’-(2-hydroxy-1-hydroxymethyl-ethyl)- 2,4,6-triiodo-isophthalamide (WO2002044125)
5-Amino-N,N’-bis-(2,3-dihydroxy-propyl)-2,4,6-triiodo-N,N’-dimethyl- isophthalamide
5-Amino-N-(2,3-dihydroxy-propyl)-N’-(2-hydroxy-ethyl)-2,4,6-triiodo-is ophthalamide (WO 8700757)
The compounds of formulas (IVa) and (IVb), may be prepared by acylation of the corresponding compounds having free amino groups. In this reaction, hydroxyl groups in the substituents R may also be protected by acylation.
Acylation may be effected by any convenient method, e.g. by use of activated formic acid such as mixed anhydrides which can prepared by a variety of methods described in the literature.
A convenient method of preparing mixed anhydrides is to add a carboxylic acid anhydride to an excess of formic acid under controlled temperature. It is also possible to make mixed anhydrides by addition of a carboxylic acid chloride to a solution of a formic acid salt. Formyl-mixed anhydrides may include acetyl, isobutyryl, pivaloyl, benzoyl etc.
In the present implementation acetic-formic mixed anhydride is employed. To an excess of cooled pre-prepared acetic-formic mixed anhydride is added a 5-amino- monomer and the mixture is stirred overnight. The mixture is concentrated in vacuo and may be used directly in the alkylation step as described in the experimental section (procedure B) or alternatively the O-acylated groups may be hydrolysed prior to alkylation as described in the experimental section (procedure A). Hydrolysis is conveniently performed in aqueous basic media as exemplified in the experimental section or may alternatively be effected by alcoholysis e.g. as described in WO1997000240.
It is also possible to dissolve the 5-aminomonomer in formic acid and subsequently add the carboxylic acid anhydride but in order to reduce unwanted acylation it is preferred to prepare the mixed anhydride separately and subsequently mix this with the 5-aminomonomer as described above.
Experimental
Example 1
5,5′-(2-hvdroxypropane-1 ,3-diyl)bis(formylazanediyl)bis(N1,N3-bis(2,3- dihvdroxypropyl)-2.4,6-triiodoisophthalamide)
Procedure A:
1 a) N,N’-Bis-(213-dihvdroxy-propyl)-5-formylamino-2,4,6-triiodo-isophthalamide Formic acid (300 ml) was charged in a dry 1000 ml flask fitted with a dropping funnel, stir bar, thermometer and a gas inlet. The acid was cooled on an ice bath under a nitrogen blanket and acetic anhydride (144.8 g, 1.418 mol) was added drop wise at a rate so that the temperature did not exceed 2.5 C. After complete addition, the ice bath was removed and the temperature was allowed to reach 10 °C. The mixture was again ice cooled and 5-amino-N,N’-bis(2,3-dihydroxypropyl)-2,4,6- triiodo-isophthalamide (100 g, 141.8 mmol) was added over 5 minutes and the mixture was left stirring over night while attaining ambient temperature. The mixture was evaporated to dryness and methanol (300 ml) and water (300 ml) was added. 2 M potassium hydroxide was added until all material was in solution and a stable pH 12.5 was attained. The methanol was removed in vacuo. The mixture was neutralized with 4 M HCI and a slow precipitation started. 300 ml water was added and the product was precipitated over night. The precipitate was collected and rinsed with a small amount of water and dried on filter to a moist cake and further dried in vacuo to yield 84.8 g ( 81.5 %) of N,N’-bis-
(2,3-dihydroxy-propyl)-5-formylamino-2,4,6-triiodo-isophthalamide.
1H-NMR 500 MHz (solvent: D2O, ref. H2O=4.8 ppm, 25 0C): 8.35 and 8.05 ppm (2s,
1 H), 3.94 ppm (m, 2H), 3.67 ppm (m, 2H), 3.55 ppm (m, 2H), 3.45 ppm (m, 2H),
3.34 ppm (m, 2H).
LC-MS (column Agilent Zorbax SB-Aq 3.5 μm 3.0 x 100 mm, solvents: A = water/ 0.1 % formic acid and B = acetonitrile/ 0.1% formic acid; gradient 0-30 % B over 20 min; flow 0.3 ml/ min, UV detection at 214 and 254 nm, ESI-MS) gave two peaks centred at 5.5 minutes with m/z (M + H+) 733.828, m/z (M + NH4+) 750.855, m/z (M + Na+) 755.817 corresponding to the structure.
1 b) 5,5′-(2-hvdroxypropane-1 ,3-diyl)bis(formylazanediyl)bis(N1,N3-bis(2,3- dihvdroxypropyl)-2,4,6-triiodoisophthalamide)
Potassium hydroxide (1.07 g) was dissolved in water (6.9 ml) and methanol (3.4 ml) in a 50 ml round bottomed flask fitted with a magnetic stir bar. Boric acid (0.41 g, 6.6 mmol) and N,N’-bis-(2,3-dihydroxy-propyl)-5-formylamino-2,4,6-triiodo- isophthalamide (7.0 g, 9.56 mmol) was added to the stirred solution.. Epichlorohydrin (260 ul, 3.32 mmol) was added to the solution and a pH electrode was fitted in the flask and the pH was maintained at pH 12.7 by drop wise addition of 4 M potassium hydroxide for 4 h. At this point, the mixture was left stirring over night. The pH was adjusted with 4 M hydrochloric acid to pH 4 and the methanol was removed in vacuo. The remaining aqueous solution was diluted with water (75 ml) and treated with ion exchangers (AMB200C and IRA67) to zero conductivity. The ion exchangers were removed by filtration and rinsed with water and the combined aqueous filtrates were freeze dried. The crude product was purified by preparative HPLC (column Phenomenex Luna C18 10 μm solvents: A = water and B = acetonitrile; gradient 05-20 % B over 60 min. After freeze drying 3.80 g of 5,5′- (2-hydroxypropane-1 ,3-diyl)bis(formylazanediyl)bis(N1,N3-bis(2,3-dihydroxypropyl)- 2,4,6-triiodoisophthalamide) (74.8 % yield) was obtained.
1H-NMR 500 MHz (solvent: D2O, ref. H2O=4.8 ppm, 25 0C): 8.34 and 8.08 ppm (m, 2 H), 2.80-4.80 ppm (m 26 H). LC-MS TOF; 1522.68 m/z (M + H+), 1544.66 m/z (M + Na+).
…………
New patent
Process for the preparation of 1-formylamino-3,5-bis(2,3-bis(formyloxy)propan-1-ylcarbamoyl)-2,4,6-trioodobenzene, used as a key intermediate in the preparation of ioforminol. Also claims a process for the preparation of ioforminol, useful in X-ray imaging. GE Healthcare is developing ioforminol (GE-145; AN-113111) as an iv contrast agent (Phase 2). See WO2013104690 claiming X-ray imaging contrast media with low iodine concentration and X-ray imaging process. Also see concurrently published WO2014052092 claiming preparation of ioforminol. Appears to be the first filing from Medi-Physics on this compound.
……………
The most preferred iodinated agents are;
Diatrizoic acid
loxaglinic acid
loversol
lodixanol
lomeprol
lobitriol
The most preferred chelates are:
Gadopentetate
Ňadoversetamide
Gadoxetinic acid
Monday, 7 April 2014
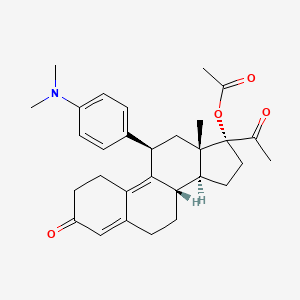
Ulipristal acetate for emergency contraception

Ulipristal acetate
17alpha-Acetoxy-11beta-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
(8S,11S,13S,14R,17R)-17-Acetoxy-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
126784-99-4 CAS
Ella, CDB 2914, 126784-99-4, CDB-2914, VA2914, Ulipristal acetate (USAN), Ulipristal acetate [USAN]
Molecular Formula: C30H37NO4 Molecular Weight: 475.61908
17α-acetoxy-llβ-(4-N, N-dimethylaminophenyl)-19-norpregna-4, 9 - dien-3 ,20-dione,
NA-2914,
CDB-2914
HRP-2000
PGL-4001
RTI-3021-012
UPA-UF
VA-2914
HRP-2000
PGL-4001
RTI-3021-012
UPA-UF
VA-2914
Ulipristal acetate is effective as an emergency contraceptive for up to 120 hours after unprotected intercourse. Because ulipristal is available only via prescription, it may be covered by insurance. However, the additional factors of travel expenses and time to make and attend a physician appointment must be taken into account when considering use of ulipristal as an emergency contraceptive. Due to the similarity of its structure to mifepristone, controversy regarding ulipristal's mechanism of action has arisen
DETAILS
CHECK OUT NEW PATENTS BELOW NEW PATENTS IN 2014
WO-2014050105 Amorphous ulipristal acetate, ASKA Pharmaceutical Co Ltd
WO-2014050106 Crystalline polymorphic form of ulipristal acetate
WO-2014050107 Crystalline polymorphic form of ulipristal acetate
WO-2014050105 Amorphous ulipristal acetate, ASKA Pharmaceutical Co Ltd
WO-2014050106 Crystalline polymorphic form of ulipristal acetate
WO-2014050107 Crystalline polymorphic form of ulipristal acetate
Ulipristal acetate (trade name EllaOne in the European Union, Ella in the U.S. for contraception,[1] and Esmya for uterine fibroid) is a selective progesterone receptor modulator (SPRM).
Medical uses
Emergency contraception
For emergency contraception[2] a 30 mg tablet is used within 120 hours (5 days) after an unprotected intercourse or contraceptive failure.[3] It has been shown to prevent about 60% of expected pregnancies,[4] and prevents more pregnancies than emergency contraception with levonorgestrel.[5] Ulipristal acetate is available by prescription for emergency contraception in over 50 countries, with access through pharmacists without a prescription being tested in the United Kingdom.[6][7][8][9] Emergency contraception (EC) is a woman's second chance for primary prevention of pregnancy.
A reproductive-age woman is a candidate for emergency contraception if she seeks care within 120 hours of unprotected intercourse (UPI), which is the window of pregnancy risk associated with a given act of intercourse based upon the estimated lifespan of sperm in the genital tract (Wilcox et al, 1995). Current hormonal methods of emergency contraception prevent at least half of expected pregnancies if taken within 72 hours of UPI (Von Hertzen et al, 1998).
Levonorgestrel at a total dose of 1.5 mg (taken in a single dose or two 0.75 mg doses 12 hours apart) is the current standard for hormonal emergency contraception and is licensed for use up to 72 hours after UPI. Clinical trials involving levonorgestrel used for emergency contraception more than 72 hours after intercourse do not conclusively establish efficacy rates because of insufficient sample size. Nevertheless, these studies reveal a trend towards markedly higher failure rates when levonorgestrel is taken 48 hours or more after unprotected intercourse (von Hertzen et al, 1998; Von Hertzen et al, 2002).
This trend may be explained by levonorgestrel mode of action for emergency contraception. Levonorgestrel acts by interfering with the LH peak but does not appear to interfere with the ovulatory process when taken close to ovulation, a time when intercourse is most likely to lead to fertilization (Croxatto et al, 2004; Marions et al, 2004; Wilcox et al, 2004). For a woman who presents for emergency contraception more than 72 hours after intercourse, the only currently available method proven to be highly effective is insertion of a copper contraceptive intra-uterine device (IUD). However, IUDs are not widely available in many countries and insertion can only be performed by a trained clinician. Furthermore, many women decline IUD insertion as a method of emergency contraception because the procedure is invasive, is relatively expensive and has a risk of complications including uterine perforation on insertion (Grimes et al, 2004). Additionally, many women seeking emergency contraception are not seeking a long acting contraceptive method.
There is, therefore, a need for a new hormonal emergency contraceptive that can be used and is highly effective up to 120 hours after UPI. Ulipristal acetate (also known as CDB-2914) is a selective progesterone receptor modulator that inhibits or delays ovulation in a dose-dependent fashion (Stratton et al, 2000). In a double-blind non-inferiority trial, ulipristal acetate was shown to be as efficacious as levonorgestrel for preventing pregnancy when used within 72 hours of UPI (Creinin et al, 2006). Moreover, study data suggest improved efficacy in preventing pregnancy from 48 to 72 hours when levonorgestrel efficacy markedly wanes. ulipristal acetate for use in providing post coital contraception in a female subject between about 3 to about 5 days, or between about 72 to about 120 hours, after unprotected intercourse.
A subject of the invention is thus a method for providing post coital contraception in a female subject, comprising providing the subject with a therapeutically effective amount of ulipristal acetate, between about 3 to about 5 days, or between about 72 to about 120 hours, after unprotected intercourse. It is further provided a kit comprising i) a dosage form comprising ulipristal acetate and ii) a printed matter stating that ulipristal acetate may be taken within 120 hours or 5 days after unprotected intercourse Any woman of reproductive age may need post-coital or emergency contraception at some point to avoid an unintended pregnancy. It is meant to be used in situations of unprotected intercourse, such as: when no contraceptive has been used;
when there is a contraceptive failure or incorrect use, including: - condom breakage, slippage, or incorrect use; - non-compliance with dosage regimen for combined oral contraceptive pills; - non-compliance with dosage regimen for progestogen-only pill (minipill); - more than two weeks late for a progestogen-only contraceptive injection (depot- medroxyprogesterone acetate or norethisterone enanthate); - more than seven days late for a combined estrogen-plus-progestogen monthly injection; - dislodgment, delay in placing, or early removal of a contraceptive hormonal skin patch or ring; - dislodgment, breakage, tearing, or early removal of a diaphragm or cervical cap; - failed coitus interruptus (e.g., ejaculation in vagina or on external genitalia); - failure of a spermicide tablet or film to melt before intercourse; - miscalculation of the periodic abstinence method or failure to abstain on fertile day of cycle; - IUD expulsion; or in cases of sexual assault when the woman was not protected by an effective contraceptive method.
Uliprisnil acetate, originally developed at the Research Triangle Institute, is a selective progesterone receptor modulator (SPRM) first launched in the E.U. in 2009 by HRA Pharma as emergency contraception within 120 hours (5 days) of unprotected sexual intercourse or contraceptive failure. The company filed for approval of this indication in the U.S. in 2009 and approval was obtained in 2010. In 2012, the product was approved in the E.U. for the pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. First E.U. commercialization took place in Germany in March 2012 followed by the U.K. in April. The compound is being developed in phase II clinical trials at the National Institutes of Health (NIH) for the treatment of uterine fibroids and premenstrual syndrome (PMS). Two formulations of uliprisnil are in early clinical trials at the Population Council for the prevention of pregnancy: a vaginal ring and an intrauterine delivery system (IUS). Watson conducted phase III clinical studies for the treatment of women with anemia associated with uterine leiomyoma, however the development has been discontinued.
Uliprisnil acetate is a well-known steroid that possesses antiprogestational and antiglucocorticoid activity. In preclinical studies, the growth of lead follicles exposed to a midfollicular dose of the compound was delayed in a dose-related fashion, indicating that the compound may have an additional mechanism of action involving progesterone or estrogen antagonism.
In 2007, uliprisnil acetate was licensed to PregLem by HRA Pharma in Europe for the treatment of gynecological disorders excluding contraception. A license for North American was granted to HRA in 2010. In 2010, the compound was licensed to Watson (now Actavis) by HRA Pharma for the commercialization in the U.S. for use as emergency contraception. Also in 2010, Watson (now Actavis) obtained a license to uliprisnil for the treatment of uterine fibroids. In 2011, the product was licensed to Gedeon Richter by HRA Pharma for marketing and distribution in China, Russia and (Commonwealth of Independent States) CIS republics for the treatment of uterine myoma.
Treatment of uterine fibroids
Ulipristal acetate is used for pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age in a daily dose of a 5 mg tablet.[10] Treatment of uterine fibroids with ulipristal acetate for 13 weeks effectively controlled excessive bleeding due to uterine fibroids and reduced the size of the fibroids.[11][12][13] Two intermittent 3-month treatment courses of ulipristal acetate 10 mg resulted in amenorrhea at the end of the first treatment course in 79.5%, at the end of the second course in 88.5% of subjects. Mean myoma volume reduction observed during the first treatment course (−41.9%) was maintained during the second one (−43.7%).[10
Adverse effects
Common side effects include abdominal pain and temporary menstrual irregularity or disruption. Headache and nausea were observed under long-term administration (12 weeks), but not after a single dose.[3]
Interactions
Ulipristal acetate is metabolized by CYP3A4 in vitro. Ulipristal acetate is likely to interact with substrates of CYP3A4, like rifampicin, phenytoin, St John's wort, carbamazepine or ritonavir, therefore concomitant use with these agens is not recommended.[10][14] It might also interact with hormonal contraceptives and progestogens such as levonorgestrel and other substrates of the progesterone receptor, as well as with glucocorticoids.[10]
Contraindications
Ulipristal acetate should not be taken by women with severe liver diseases[3] because of its CYP mediated metabolism. It has not been studied in women under the age of 18.[15]
Pregnancy
Unlike levonorgestrel, and like mifepristone, ulipristal acetate is embryotoxic in animal studies.[16] Before taking the drug, a pregnancy must be excluded.[3] The EMA proposed to avoid any allusion to a possible use as an abortifacient in the package insert to avert off-label use.[17] It is unlikely that ulipristal acetate could effectively be used as an abortifacient, since it is used in much lower doses (30 mg) than the roughly equipotent mifepristone (600 mg), and since mifepristone has to be combined with a prostaglandin for the induction of abortion.[18] However, data on embryotoxicity in humans are very limited, and it is not clear what the risk for an abortion or for teratogenicity (birth defects) is. Of the 29 women studied who became pregnant despite taking ulipristal acetate, 16 had induced abortions, six had spontaneous abortions, six continued the pregnancies, and one "was lost to follow-up".[19]
Lactation
It is not recommended to breast feed within 36 hours of taking the drug since it is not known whether ulipristal acetate or its metabolites are excreted into the breast milk.[3][20]
Pharmacokinetics
In animal studies, the drug was quickly and nearly completely absorbed from the gut. Intake of food delays absorption, but it is not known whether this is clinically relevant.[21] Ulipristal acetate is metabolized in the liver, most likely by CYP3A4, and to a small extent by CYP1A2 and CYP2D6. The two main metabolites have been shown to be pharmacologically active, but less than the original drug. The main excretion route is via the faeces.[22]
Pharmacodynamics
As a SPRM, ulipristal acetate has partial agonistic as well as antagonistic effects on the progesterone receptor. It also binds to the glucocorticoid receptor, but has no relevant affinity to the estrogen, androgen and mineralocorticoid receptors.[23] Phase II clinical trials suggest that the mechanism might consist of blocking or delaying ovulation and of delaying the maturation of the endometrium.[24]
History
Ulipristal acetate was granted marketing authorization by the European Medicines Agency (EMA) in March 2009.[25] The U.S. Food and Drug Administration approved the drug for use in the United States on 13 August 2010,[26] following the FDA advisory committee's recommendation.[27][28] Watson Pharmaceuticals announced the availability of ulipristal acetate in the United States on 1 December 2010, in retail pharmacies, clinics, and one on-line pharmacy, KwikMed.[29] Amorphous ulipristal acetate. ASKA is developing ulipristal acetate in Japan under license from HRA Pharma for the treatment of uterine fibroids and for emergency contraception. In March 2014, it was in phase II for both indications (in Japan). Also see the co-published WO2014050106 and WO2014050107. Crystalline polymorphic form C of ulipristal acetate.
Also claims its method of preparation. Appears to be the first filing from the assignee on this API, which was developed by HRA Pharma under license from the RTI, indicated in the US as an emergency contraceptive for prevention of pregnancy. In May 2011, ASKA signed an exclusive licensing agreement with HRA Pharma to develop and commercialize the API . In November 2013, ASKA had begun phase II development for emergency contraception and uterine fibroids [1339186] in Japan. Also see concurrently published WO2014050105 and WO2014050107. Crystalline polymorphic form B of ulipristal acetate. Also claims process for the preparation and composition comprising the same. Useful for the treatment of uterine leiomyoma.
Appears to be the first filing from the assignee on this API, see concurrently published WO2014050105 and WO2014050106. The drug was developed by HRA Pharma under license from the RTI, indicated in the US as an emergency contraceptive for prevention of pregnancy. In May 2011, ASKA signed an exclusive licensing agreement with HRA Pharma to develop and commercialize the API In November 2013, ASKA had begun phase II development in Japan for emergency contraception and uterine fibroids Buccal forms or devices are also useful, such as those described in U.S. patent application 20050208129 , herein incorporated by reference. U.S. patent application 20050208129 describes a prolonged release bioadhesive mucosal therapeutic system containing at least one active principle, with an active principle dissolution test of more than 70% over 8 hours and to a method for its preparation.
Said bioadhesive therapeutic system comprises quantities of natural proteins representing at least 50% by weight of active principle and at least 20% by weight of said tablet, between 10% and 20% of a hydrophilic polymer, and compression excipients, and comprising between 4% and 10% of an alkali metal alkylsulphate to reinforce the local availability of active principle and between 0.1 % and 1% of a monohydrate sugar. 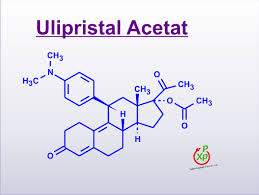
Ulipristal acetate, formerly known as CDB-2914, designates within the context of this application 17α-acetoxy-11β-[4-N,N-dimethylamino-phenyl)-19-norpregna-4,9-diene-3,20-dione, represented by formula I:
Ulipristal acetate, and methods for its preparation, are described e.g., in U.S. Pat. Nos. 4,954,490; 5,073,548, and 5,929,262, as well as in international patent applications WO2004/065405 and WO2004/078709. Ulipristal acetate possesses antiprogestational and antiglucocorticoidal activity, and has been proposed for contraception, in particular for emergency contraception, and for the therapy of various hormonal diseases.
(Steroids, 2000,65, 395 ~ 400; US5929262A; CN1298409A; CN101466723A). Reaction is as follows:
Properties of this compound are further described in Blithe et al, Steroids. 2003 68(10-13):1013-7. So far, clinical trials have been conducted using oral capsules of ulipristal acetate (Creinin et al, Obstetrics & Gynecology 2006; 108:1089-1097; Levens et al, Obstet Gynecol. 2008, 111(5):1129-36). In order to increase the properties and clinical benefit of this molecule, there is a need for improved formulations thereof
- It is a well-known steroid, more specifically a 19-norprogesterone, which possesses antiprogestational and antiglucocorticoidal activity. This compound, and methods for its preparation, are described in U. S. Patent Nos. 4,954, 490,5 , 073,548 , and 5,929, 262 , and international patent applications WO2004/065405 andWO2004/078709 . Properties of this compound are further described in Blithe et al, 2003.
- It is now proposed to use ulipristal acetate or a metabolite thereof for treating uterine fibroids, more particularly for reducing or stopping bleeding in a patient afflicted with uterine fibroids, reducing the size of uterine fibroids and/or reducing uterine volume More particularly the inventors have shown in a randomized, placebo-controlled, double blinded, parallel trial, that ulipristal acetate significantly reduces fibroid volume after 3 months, and stops bleeding
- Ulipristal acetate or a metabolite thereof alleviates symptoms of uterine fibroids, including bleeding, pelvic pain, pressure.
- Ulipristal acetate or a metabolite thereof is useful for preventing or treating anemia in patients afflicted with uterine fibroids.
- It is also useful for preventing or treating leiomyosarcomas and for preventing dissemination of uterine fibroids to other organs.
..................
synthesis
The United States Of America As Represented By The Department Of Health And Human Services 
 EXAMPLE 7 The Preparation of the Compound of Formula (I) (17α-Acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione) From the Compound of Formula (VIII) 340 mL of acetic acid (5.92 mol) were added to a well stirred mixture containing 834 mL of trifluoroacetic anhydride (5.92 mol) in 2,300 mL of methylene chloride under argon. After stirring for 30 minutes at room temperature, 51.3 g of p-toluenesulfonic acid (0.26 mol) were added, and the mixture was chilled to 0 methylene chloride solution containing 128.3 g of the compound of formula (VIII) (0.30 mol) were added, and the reaction mixture was stirred at 0 cautious addition of a 4.5N potassium carbonate solution until the pH was in the range of 7.0-7.5. The reaction mixture was diluted with water and extracted with methylene chloride. The methylene chloride extracts were washed with water and brine, combined, and dried over sodium sulfate.
EXAMPLE 7 The Preparation of the Compound of Formula (I) (17α-Acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione) From the Compound of Formula (VIII) 340 mL of acetic acid (5.92 mol) were added to a well stirred mixture containing 834 mL of trifluoroacetic anhydride (5.92 mol) in 2,300 mL of methylene chloride under argon. After stirring for 30 minutes at room temperature, 51.3 g of p-toluenesulfonic acid (0.26 mol) were added, and the mixture was chilled to 0 methylene chloride solution containing 128.3 g of the compound of formula (VIII) (0.30 mol) were added, and the reaction mixture was stirred at 0 cautious addition of a 4.5N potassium carbonate solution until the pH was in the range of 7.0-7.5. The reaction mixture was diluted with water and extracted with methylene chloride. The methylene chloride extracts were washed with water and brine, combined, and dried over sodium sulfate.

 EXAMPLE 7 The Preparation of the Compound of Formula (I) (17α-Acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione) From the Compound of Formula (VIII) 340 mL of acetic acid (5.92 mol) were added to a well stirred mixture containing 834 mL of trifluoroacetic anhydride (5.92 mol) in 2,300 mL of methylene chloride under argon. After stirring for 30 minutes at room temperature, 51.3 g of p-toluenesulfonic acid (0.26 mol) were added, and the mixture was chilled to 0 methylene chloride solution containing 128.3 g of the compound of formula (VIII) (0.30 mol) were added, and the reaction mixture was stirred at 0 cautious addition of a 4.5N potassium carbonate solution until the pH was in the range of 7.0-7.5. The reaction mixture was diluted with water and extracted with methylene chloride. The methylene chloride extracts were washed with water and brine, combined, and dried over sodium sulfate.
EXAMPLE 7 The Preparation of the Compound of Formula (I) (17α-Acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione) From the Compound of Formula (VIII) 340 mL of acetic acid (5.92 mol) were added to a well stirred mixture containing 834 mL of trifluoroacetic anhydride (5.92 mol) in 2,300 mL of methylene chloride under argon. After stirring for 30 minutes at room temperature, 51.3 g of p-toluenesulfonic acid (0.26 mol) were added, and the mixture was chilled to 0 methylene chloride solution containing 128.3 g of the compound of formula (VIII) (0.30 mol) were added, and the reaction mixture was stirred at 0 cautious addition of a 4.5N potassium carbonate solution until the pH was in the range of 7.0-7.5. The reaction mixture was diluted with water and extracted with methylene chloride. The methylene chloride extracts were washed with water and brine, combined, and dried over sodium sulfate.
Evaporation of the solvent gave the acetate of formula (I) as a thick syrup. The above syrup was dissolved in 300 mL of isopropyl alcohol and evaporated. The dissolution and evaporation were repeated three times. Finally, the remaining solid, which retained isopropyl alcohol as solvent of recrystallization, was dissolved in ethyl acetate and evaporated to give a stable foam. The foam was quickly dissolved in ether, and this solution was set aside to crystallize. The solid that formed was collected by filtration, washed with ether, and dried in vacuo to yield 105.7 g of the compound of formula (I) as yellow crystals in 75% yield;
m.p. 183-185 1735 and 1714(--C═O), 1664 and 1661 (conjugated --C═O), 1563, 1518, 1441, 1351, 1305, 1252, 1203, 1171; NMR (CDCl.sub.3) δ0.38 (s, 18-CH.sub.3), 2.10 (s, 17-OAc), 2.14 (s, 21-CH.sub.3), 2.92 (s, --N(CH.sub.3).sub.2, 4.44 (d, C-11 H), 5.83 (br. s, C-4 H), 6.71 and 7.07 (d, aromatic H); MS(EI) m/z (relative intensity) 475(M.sup.+, 41), 134(18), 121 (100). Analysis calculated for C.sub.30 H.sub.37 NO.sub.4 : C, 75.76; H, 7.84; N, 2.94. Found. C, 75.80; H 7.96; N, 3.09.
.................
EXAMPLE 1 Preparation of 17α-aceto-d-llβ-(4-N, N-dimetüaminofeniI)-19-norpregna-4 ,9-dien-3,20-dione [VA-2914] Raw Were charged 38.5 g of 3,3 - (l ,2-etanodioxi)-5α-hydroxy-llβ-(4-N, N-dimethylaminophenyl)-17α-acetoxy-19-norpregna-9-en-20-one [carbinol acetate] purified in a flask under nitrogen atmosphere at a temperature between 20 ° C and 22 ° C, and added 385 ml of deionized water and 17.91 g of HKSO. The resulting suspension was stirred until complete dissolution, for about 4 hours. The end of the reaction was determined by thin layer chromatography (TLC). Then added 3.85 g of neutral Al 2 O 3, stirred for 30 minutes, the suspension was filtered and the insolubles were washed with 38.5 ml of deionized water. To the filtrate were added 325 ml of ethyl acetate and the pH was adjusted to a constant value between 7.0 and 7.2 with sodium bicarbonate solution to 7% w / v. The phases were allowed to decant for 15 minutes and, after checking the absence of the final product therein by means of TLC, the phases were separated, discarding the aqueous phase. The resultant organic phase was added 192.5 ml of deionized water, stirred for 10 minutes and the phases were allowed to decant for 15 minutes.
After verifying the absence of aqueous phase final product by TLC, the phases were separated, discarding the aqueous phase. The resulting organic phase was concentrated under vacuum to a residue and obtained approximately 28 g of 17α-acetoxy-llβ-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-dien-3 ,20-dione [NA -2914] raw. EXAMPLE 2Isopropanol hemisolvate obtaining 17α-acetoxy-llβ-(4-Ν, Ν-dimethylaminophenyl)-19-norpregna-4 ,9-dien-3 ,20-dioneThe crude 17α-acetoxy-l lβ-(4-Ν, Ν-dimethylaminophenyl)-19-norpregna-4 ,9-dien-3,20-dione obtained in Example 1 was added 2 x 38.5 ml isopropanol concentrating vacuum to a residue both times. The finally obtained solid was added 77 ml of isopropanol and heated until dissolved. Then allowed to cool to a temperature between 0 ° C and 5 ° C, and the temperature was maintained for 1 h. The resulting suspension was filtered and the cake washed with cold isopropanol.
The yield achieved was 96% molar (5.5% isopropanol content). Isopropanol hemisolvate obtemdo NA-2914 has been characterized by IR spectroscopy, DSC and XRD, as indicated in the description, and has the characteristics indicated therein and shown in Figures 1-3.
................
A new and efficient method for the synthesis of Ulipristal acetate


In this study, we describe another new and efficient route for preparing Ulipristal acetate. The 1,4-addition compound 5 was greatly improved after the starting material ketone 1 was underwent epoxidation, cyanation, hydroxyl group protection and Grignard addition. The synthetic procedure is only 6 steps and the total yield is about 27.4%, which is much suitable for industrial process.
We have succeeded in finding another convenient and efficient synthetic route for the synthesis of Ulipristal acetate with a good yield.
•The yield of 11β-substituted isomer was greatly improved.
•The 17β-carbonitrile compound was obtained with high purity after the reaction.
•The yield of once Grignard addition dione was greatly improved.
•These synthetic procedures are much suitable for industrial process.
................
Volume 78, Issues 12–13, 11 December 2013, Pages 1293–1297
http://www.sciencedirect.com/science/article/pii/S0039128X13002122
We set out to describe a new and efficient route for preparing Ulipristal acetate with a good yield. The selected epoxidization conditions gave out 80% of 5α,10α-epoxide 2a in the two diastereoisomers which greatly improved the yield of 11β-substituted isomer 4a. And phenyl–sulfinyl compound 6 was synthesized from ketone 5 directly treated with phenylsulfenyl chloride in the presence of triethylamine. These synthetic procedures is only 8 steps, less than currently reported in the literature, but more suitable for industrial process.

...........
http://www.google.com/patents/CN103145787A?cl=en
Reaction is as follows:
...........
WO2013063859A1
http://www.google.com/patents/WO2013063859A1?cl=en
Preparation of related reports Uli Division acetate compounds as follows:
1, U.S. Patent US4954490 methods, (see Reaction Scheme 1),
The method is based on the 3 - methoxy -19 - norpregn-1, 3,5 (10), 17 (20) - tetraene as a starting material, in turn by the addition, oxidation, reduction, hydrolysis, addition and elimination, oxidation of 17-hydroxy-19 - norpregn left the -4,9 - 31 women -3, 20 - dione (Compound V2), and then condensed by ethylene glycol, epoxidized-chloroperbenzoic acid, Bonus format, acid hydrolysis, acetylation of 10-step reaction by Uli acetate SECRETARY (Compound 1), and a melting point of 118-121 ° C the product was obtained by recrystallization with methanol ^. Due to the method, the step length, but difficult to obtain a starting material, the complexity of the reaction conditions, the required intermediate product was purified by column chromatography, the total yield is only 0.62%, Gao costs, the instability of the resulting product is not suitable pharmaceutically acceptable. And is not suitable for industrial production.
Reaction Formula I:
2, U.S. Patent No. US5929262 discloses his method Another method for preparing acetic acid Uli Division (see Reaction Scheme II), the reaction of formula II:
The method is based on 3,3 - ethylenedioxy-17) 8 - cyano -19 - norpregn -5 (10)-9 (11) - dien-17 alcohol (compound III) as a starting material, , first with dimethyl chloromethyl silane protected hydroxy, and then at the cryogenic-70Ό obtained by acid hydrolysis with the DBB / LI reagents After the reaction, a condensation reaction with ethylene glycol ketal, epoxy reaction, then the format of the reaction, The acid hydrolysis reaction and the acetylation reaction to obtain the target object and sequentially by treatment with isopropanol, ethyl acetate and crystallized from ether to obtain a yellow product with a melting point of 183-185Ό. The method expensive starting materials prices, harsh reaction conditions, need to be ultra-low temperature and water and oxygen reaction, high cost of low yield (total yield of about 14%), and therefore not suitable for industrial production.
3, World Patent WO2004078709 discloses a method for preparing (see Reaction Scheme III), the method the Πα hydroxy _19_ norpregn _ 4, 9 (10) _ diene-_ _ 3, 17-dione (Compound V2 ), followed by acetylation of 3 - bit carbonyl condensation, epoxy, Bonus format, acid hydrolyzed to give the target. Although the steps are shorter, but a starting material is from Compound VI was prepared by hydrolysis under acidic conditions to obtain a total yield of about 11.8% (starting from the compound VI operator), the actual reaction step is longer, lower yield, higher cost not suitable for industrial production.
In this method, 3,3 - ethylenedioxy -19 - norpregn -5 (10), 9 (11) - dien-17 - one (referred to as 3 - ketal compound II) as a starting material, by the addition of acetylene, benzene sub-sulfonyl chloride, and then by hydrolysis of sodium methoxide, acid hydrolysis, condensation of ethylene glycol, epoxy, Grignard reaction, acid hydrolysis and acetylation reaction of 9-step reaction to obtain a target object, isopropoxy alcohol crystallization with ethanol and water was heated at 70 ° C after 14h excluding solvate crystal. The method uses a greater risk of acetylene and odor of benzene times sulfonyl chloride, especially benzene times, unstable sulfonyl chloride, easy storage, decomposition of impurities involved in the reaction leads to a low yield, and benzene of times sulfonyl chloride of environmental pollution Further crystallization prolonged heating will produce new impurities, the total yield of 13.8% -15.8%, high cost, is not suitable for industrial production.
The existing methods, the methods 1, 2 and 4 are related to the preparation of compound VI, and also the starting materials in Method 3 Hydrolysis of compound VI is obtained. SUMMARY OF THE INVENTION
Technical problems to be solved by the present invention is to overcome these drawbacks,, study design Uli acetate Secretary industrialization production methods.
WO2013063859A1
http://www.google.com/patents/WO2013063859A1?cl=en
A process for the preparation of formula I the Uli acetate Division his method, characterized in comprising the following reaction steps:

.........
Ulipristal Acetate intermediates(7)
|
Intermediate 1
![19-Norpregn-9-ene-3,20-dione, 11-[4-(dimethylamino)phenyl]-5,17-dihydroxy-, cyclic 3,20-bis(1,2-ethanediyl acetal), (5α,11β)-](http://www.lanospharma.com/images/19-Norpregn-9-ene-3,20-dione,%2011-[4-%28dimethylamino%29phenyl]-5,17-dihydroxy-,%20cyclic%203,20-bis%281,2-ethanediyl%20acetal%29,%20%285%CE%B1,11%CE%B2%29-.gif)
Intermediate
19-Norpregn-9-ene-3,20-dione, 11-[4-(dimethylamino)phenyl]-5,17-dihydroxy-, cyclic 3,20-bis(1,2-ethanediyl acetal), (5α,11β)-
Synonyms
3,3,3,20,20-Bis(ethylene-dioxy) -5α, 17α-dihydroxy-11β-[4-(N,N-dimethylamino)-phenyl]-19-norpregna-9(11)-ene
CAS No.
126690-41-3
Molecular Formula
C32H45NO6
Molecular Weight
539.32
Intermediate 2

Intermediate
Gestadene
Synonyms
19-Norpregna-4,9-diene-3,20-dione,17-hydroxy-
CAS No.
14340-01-3
Molecular Formula
C20H26O3
Molecular Weight
314.42
Intermediate 3
![19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-[4-(methylamino)phenyl]-, (11β)-](http://www.lanospharma.com/images/19-Norpregna-4,9-diene-3,20-dione,%2017-%28acetyloxy%29-11-[4-%28methylamino%29phenyl]-,%20%2811%CE%B2%29-.gif)
Intermediate
19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-[4-(methylamino)phenyl]-, (11β)-
Synonyms
N/A
CAS No.
159681-66-0
Molecular Formula
C29H35NO4
Molecular Weight
461.26
Intermediate 4

Intermediate
11β-[4-(N,N-dimethylamino)-phenyl]-17α-hydroxy-19-norpregna-4,9-diene-3,20-dione
Synonyms
CDB3236; Deacetyl CDB 2914; Ulipristal
CAS No.
159811-51-5
Molecular Formula
C28H35NO3
Molecular Weight
433.26
Intermediate 5

Intermediate
17-Hydroxy-19-norpregna-5(10),9(11)-diene-3,20-dione cyclic 3-(1,2-ethanediyl acetal)
Synonyms
19-Norpregna-5(10),9(11)-diene-3,20-dione,17-hydroxy-, cyclic 3-(1,2-ethanediyl acetal)
CAS No.
42982-49-0
Molecular Formula
C22H30O4
Molecular Weight
358.47
Intermediate 6

Intermediate
19-Norpregn-9(11)-ene-3,20-dione, 5,10-epoxy-17-hydroxy-, cyclic 3,20-bis(1,2-ethanediyl acetal), (5α,10α)-
Synonyms
N/A
CAS No.
54201-83-1
Molecular Formula
C24H34O6
Molecular Weight
418.24
Intermediate 7

Intermediate
3,20-Bis(ethylenedioxy)-19-norpregna-5(10),9(11)-dien-17-ol
Synonyms
19-Norpregna-5(10),9(11)-diene-3,20-dione,17-hydroxy-, cyclic 3,20-bis(1,2-ethanediyl acetal), (5a,10a)-
CAS No.
54201-84-2
Molecular Formula
C24H34O5
Molecular Weight
402.52
............
Review
Synthetic approaches to the 2009 new drugs
Bioorganic & Medicinal Chemistry
doc.sciencenet.cn/upload/file/2011531154034454.pdf
by KCL Kevin - 2011 - Cited by 9 - Related articles
Keywords: Synthesis. New drug molecules. New chemical entities. Medicine. Therapeutic agents. a b s t r a c t .... 1153. 22. Ulipristal acetate (ellaOne®).
References
- "FDA approves ella™ tablets for prescription emergency contraception" (Press release). FDA. 13 August 2010. Retrieved 2013-06-12.
- Creinin, MD; Schlaff, W; Archer, DF; Wan, L; Frezieres, R; Thomas, M; Rosenberg, M; Higgins, J (2006). "Progesterone receptor modulator for emergency contraception: a randomized controlled trial". Obstetrics and gynecology 108 (5): 1089–97. doi:10.1097/01.AOG.0000239440.02284.45. PMC 2853373. PMID 17077229.
- "Summary of Product Characteristics: ellaOne 30 mg tablet". Retrieved 20 November 2010.
- "European Public Assessment Report for Ellaone. Summary for the public". EMA. 2009. p. 2. Retrieved 22 November 2009.
- Glasier, A. F.; Cameron, S. T.; Fine, P. M.; Logan, S. J.; Casale, W.; Van Horn, J.; Sogor, L.; Blithe, D. L.; Scherrer, B.; Mathe, H.; Jaspart, A.; Ulmann, A.; Gainer, E. (2010). "Ulipristal acetate versus levonorgestrel for emergency contraception: A randomised non-inferiority trial and meta-analysis". The Lancet 375 (9714): 555–562. doi:10.1016/S0140-6736(10)60101-8. PMID 20116841. edit
- Trussell, James; Cleland, Kelly (February 13, 2013). "Dedicated emergency contraceptive pills worldwide". Princeton: Office of Population Research at Princeton University, Association of Reproductive Health Professionals. Retrieved March 25, 2014.
- ICEC (2014). "EC pill types and countries of availability, by brand". New York: International Consortium for Emergency Contraception (ICEC). Retrieved March 25, 2014.
- HRA Pharma (March 2013). "Countries where ellaOne was launched". Paris: HRA Pharma. Retrieved March 25, 2014.
- ECEC (2014). "Emergency contraception availability in Europe". New York: European Consortium for Emergency Contraception (ECEC). Retrieved March 25, 2014. "Ulipristal acetate Emergency Contraception Pills (UPA ECPs), while available in most European countries since 2010, are not yet available in Albania, Estonia, Macedonia, Malta, Switzerland and Turkey. For now UPA ECPs are sold with a prescription in all countries, although provision without a prescription is currently being tested in the United Kingdom."
- "Summary of Product Characteristics: Esmya 5mg tablet". Retrieved 20 Febr 2014.
- Nieman, L. K.; Blocker, W.; Nansel, T.; Mahoney, S.; Reynolds, J.; Blithe, D.; Wesley, R.; Armstrong, A. (2011). "Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: A randomized, double-blind, placebo-controlled, phase IIb study". Fertility and Sterility 95 (2): 767–772.e1–772. doi:10.1016/j.fertnstert.2010.09.059. PMID 21055739. edit
- Levens, E. D.; Potlog-Nahari, C.; Armstrong, A. Y.; Wesley, R.; Premkumar, A.; Blithe, D. L.; Blocker, W.; Nieman, L. K. (2008). "CDB-2914 for Uterine Leiomyomata Treatment". Obstetrics & Gynecology 111 (5): 1129–1136. doi:10.1097/AOG.0b013e3181705d0e. PMC 2742990. PMID 18448745. edit
- Jacques Donnez; Tetyana F. Tatarchuk, Philippe Bouchard, Lucian Puscasiu, Nataliya F. Zakharenko, Tatiana Ivanova, Gyula Ugocsai, Michal Mara, Manju P. Jilla, Elke Bestel, Paul Terrill, Ian Osterloh, and Ernest Loumaye, for the PEARL I Study Group. "Ulipristal Acetate versus Placebo for Fibroid Treatment before Surgery". New England Journal of Medicine. doi:10.1056/NEJMoa1103182. PMID 22296075.
- CHMP (2009:12, 14)
- CHMP (2009:33, 43)
- CHMP (2009:16)
- CHMP (2009:41)
- RCOG (2004). The Care of Women Requesting Induced Abortion : Evidence-based clinical guideline number 7 (PDF). London: RCOG Press. ISBN 1-904752-06-3. Archived from the original on 27 February 2008.
- CHMP (2009:37)
- CHMP (2009:43)
- CHMP (2009:12, 20)
- CHMP (2009:13–14, 21)
- Attardi, B.; Burgenson, J.; Hild, S.; Reel, J. (2004). "In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone". The Journal of Steroid Biochemistry and Molecular Biology 88 (3): 277–288. doi:10.1016/j.jsbmb.2003.12.004. PMID 15120421. edit
- CHMP (2009:22–23)
- CHMP (2009). "Assessment Report for Ellaone". EMA. Retrieved 22 November 2009.
- "FDA grants approval of ella for emergency contraception" (Press release). HRA Pharma. 13 August 2010. Retrieved 2010-08-15.
- Emma Hitt (18 June 2010). "FDA Panel Gives Ulipristal Acetate Unanimous Positive Vote for Emergency Contraception Indication". Retrieved 2010-06-22.
- Harris, Gardiner (14 August 2010). "F.D.A. Approves 5-Day Emergency Contraceptive". The New York Times. Retrieved 14 August 2010.
- Watson PR (1 December 2010). "Watson Launches ella(R)(ulipristal acetate)". Retrieved 12 January 2010.\
| WO2004065405A1 | Jan 21, 2004 | Aug 5, 2004 | Crystal Pharma S A | Method of obtaining 17$g(a)-acetoxy-11$g(b)-(4-n,n-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione |
| WO2004078709A2 | Feb 13, 2004 | Sep 16, 2004 | Hyun K Kim | METHOD FOR PREPARING 17 α-ACETOXY-11β-(4-N,N-DIMETHYLAMINOPHENYL)-19-NORPREGNA-4,9-DIENE-3,20-DIONE, INTERMEDIATES THEREOF, AND METHODS FOR THE PREPARATION OF SUCH INTERMEDIATES |
| US4954490 | Jun 23, 1988 | Sep 4, 1990 | Research Triangle Institute | 11 β-substituted progesterone analogs |
| US5073548 | Apr 3, 1990 | Dec 17, 1991 | Research Triangle Institute | 11 β-substituted progesterone analogs |
| US5929262 | Mar 30, 1995 | Jul 27, 1999 | The United States Of America As Represented By The Department Of Health And Human Services | Method for preparing 17α-acetoxy-11β-(4-N, N-dimethylaminophyl)-19-Norpregna-4,9-diene-3, 20-dione, intermediates useful in the method, and methods for the preparation of such intermediates |
| US20050208129 | Apr 25, 2005 | Sep 22, 2005 | Bioalliance Pharma | Prolonged release bioadhesive therapeutic systems |
| WO2001074840A2 * | Mar 16, 2001 | Oct 11, 2001 | Carmie K Acosta | 17-alpha-substituted-11-beta-substituted-4-aryl and 21-substituted 19-norpregna 21-substituted 19-norpregnadienedione as antiprogestational agents |
| CN101466723A * | May 18, 2007 | Jun 24, 2009 | 吉瑞工厂 | Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process |
| CN102516345A * | Nov 1, 2011 | Jun 27, 2012 | 上海优拓医药科技有限公司 | Preparation method of ulipristal acetate and key intermediate thereof |
| US5929262 * | Mar 30, 1995 | Jul 27, 1999 | The United States Of America As Represented By The Department Of Health And Human Services | Method for preparing 17α-acetoxy-11β-(4-N, N-dimethylaminophyl)-19-Norpregna-4,9-diene-3, 20-dione, intermediates useful in the method, and methods for the preparation of such intermediates |
Subscribe to:
Comments (Atom)