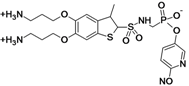
MG 96077
poster
MG96077 - MethylGene
...........http://methylgene.solocom.biz/files/2011/10/poster102.pdf .................lot of data presented
Mirati Therapeutics (USA)
Pre-Clinical for Gram-negative bacteria
Beta-Lactamase Inhibitors—Non-beta-Lactam Phosphonate-Based
Mirati Therapeutics is seeking partners to continue the development of the compound MG96077, a non-beta-lactam phosphonate-based beta-lactamase inhibitor that has shown an inhibitory profile for both class A and class C beta-lactamase enzymes [1,2].
Potent, irreversible inhibitor of serine β-lactamases that efficiently protects βlactams from hydrolysis in a variety of class
A- and class C-producing organisms-
A- and class C-producing organisms-
September 14, 2009 13:23 ET
MethylGene Presents Preclinical Data for Its Beta-Lactamase Inhibitor, MG96077, at the 49th Annual ICAAC Meeting
MONTREAL, QUEBEC--(Marketwire - Sept. 14, 2009) - MethylGene Inc. (TSX:MYG) today disclosed preclinical data for MG96077, a novel, broad spectrum, non-beta-lactam beta-lactamase inhibitor (BLI). MG96077 possesses a broad-spectrum inhibitory profile for both class A and class C beta-lactamase enzymes, including extended spectrum beta-lactamases (ESBLs). In addition, the compound overcomes resistance in beta-lactam-resistant organisms such as Pseudomonas aeruginosa. The data were presented in a poster session at the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Annual Meeting in San Francisco, California.
Poster C1-1373: Novel Beta-Lactamase Inhibitor Potentiates and Extends the Antibacterial Activity of Imipenem against Beta-Lactam-Resistant P. aeruginosa and K. pneumoniae
MG96077 was tested in combination with imipenem, a commonly-used antibiotic agent for a variety of serious infections.
A series of in vitro and in vivo preclinical studies focused on comparing the combination of MG96077 and imipenem to imipenem alone, or imipenem plus currently approved BLIs, were performed. Greater than 90 percent of imipenem-resistant clinical isolates of Pseudomonas aeruginosa and Klebsiella pneumoniae were rendered susceptible with the addition of MG96077 to imipenem. The combination of imipenem and any of the three currently approved BLIs did not achieve greater than 61 percent coverage.
Furthermore, the combination of imipenem and MG96077 in vivo demonstrated 3-6 log reduction in colony forming units (CFU) and a 100 percent survival rate in combating imipenem-resistant P. aeruginosa infections of mouse spleen and lung. The pharmacokinetic properties of MG96077 were similar to imipenem in preclinical studies with no observable drug-drug interactions.
Thus, MG96077 is a novel beta-lactamase inhibitor that restores efficacy to imipenem against a high percentage of imipenem-resistant Pseudomonas and Klebsiella strains and, therefore, may address the clinical need for antibacterial therapies with more potent coverage of resistant gram-negative organisms.
MethylGene retains exclusive rights to MG96077 and a series of related molecules. Additional data has been developed regarding MG96077 compared to other beta-lactam antibiotics, as well as other compounds in the series paired with various beta-lactam antibiotics.
"Antibiotic resistance rates are increasing among several problematic gram-negative pathogens, including P. aeruginosa, K. pneumoniae, Acinetobacter spp. and Enterobacteriaceae that are often responsible for serious hospital-acquired infections. In these studies, MG96077 appears to demonstrate activity in a variety of organisms and we look forward to further evaluation of this compound in what is a growing antibiotic market in need of novel treatments," said Donald F. Corcoran, President and Chief Executive Officer of MethylGene.
About MethylGene
MethylGene Inc. (TSX:MYG) is a publicly-traded, clinical stage, biopharmaceutical company focused on the discovery, development and commercialization of novel therapeutics with a focus on cancer. The Company's product candidates include: MGCD265, an oral, multi-targeted kinase inhibitor targeting the c-Met, VEGF, Ron and Tie-2 receptor tyrosine kinases that is in Phase I and Phase II clinical trials for cancers; MGCD290, a fungal Hos2 inhibitor being developed for use in combination with fluconazole for serious fungal infections that is in Phase I clinical studies; and MGCD0103, an oral, isoform-selective HDAC inhibitor which has been in multiple clinical trials for solid tumors and hematological malignancies and is licensed to Taiho Pharmaceutical Co. Ltd. A fourth compound discovered using MethylGene's HDAC platform, EVP-0334 - a potential cognition enhancing agent, is in a Phase I study sponsored by EnVivo Pharmaceuticals Inc. MethylGene also has a funded collaboration with Otsuka Pharmaceutical Co. Ltd. for applications in ocular diseases using the Company's proprietary kinase inhibitor chemistry. Please visit our website at www.methylgene.com.
- Martell, L.A.; Rahil, G.; Vaisburg, A.; Young, K.; Hickey, E.; Hermes, J.; Dininno, F.; Besterman, J.M. A Novel Beta-Lactamase Inhibitor Potentiates and Extends the Antibacterial Activity of Imipenem against β-Lactam-Resistant P. aeruginosa and K. pneumoniae. In Proceedings of 49th ICAAC Annual Meeting, San Francisco, CA, USA, 14 September 2009.
- Mirati Therapeutics. MG96077. Available online: http://mirati.com/other-pipeline-assets/mg96077(accessed on 9 July 2013).
- 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Annual Meeting in San Francisco, California.
Poster C1-1373: Novel Beta-Lactamase Inhibitor Potentiates and Extends the Antibacterial Activity of Imipenem against Beta-Lactam-Resistant P. aeruginosa and K. pneumoniae

No comments:
Post a Comment