World’s first biosimilar antibody is approved in Korea « New Drug Approvals:
'via Blog this'
MEDICINAL CHEMISTRY AT ITS BEST, Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, worlddrugtracker, helping millions with websites, 6 million hits on google, one lakh connections worldwide, email amcrasto@gmail.com, call +91 9323115463 India
Pages
- Home
- ABOUT ME
- FLOZIN SERIES 1/2
- SARTAN SERIES
- DRUG LEAD AND CHEMBASE
- DRUG INFO PORTAL
- Setron series
- PITANT SERIES
- VIR SERIES ..HEP C VIRUS 1/2
- SENTAN SERIES
- List of drugs 2012 FDA
- List Of drugs 2014 FDA
- GLUSTAT SERIES
- COXIB SERIES
- FLOXACIN SERIES
- Brand-Name Drug Patent Expiration Search
- Gravir series
- List of drugs 2013 FDA
- PROST SERIES
- ROLIMUS SERIES
- LIDOMIDE SERIES
Wednesday, 18 June 2014
The US Food and Drug Administration (FDA) has approved Bayer HealthCare’s Gadavist (gadobutrol) injection as the first magnetic resonance contrast agent for evaluation of breast cancer in the US « New Drug Approvals
Alternative solid-state forms of a potent antimalarial aminopyridine: X-ray crystallographic, thermal and solubility aspects « New Drug Approvals
Monday, 16 June 2014
Drug solid solutions – a method for tuning phase transformations
Drug solid solutions – a method for tuning phase transformations
Amit Delori, Pauline Maclure, Rajni M. Bhardwaj, Andrea Johnston, Alastair J. Florence, Oliver B. Sutcliffe and Iain D. H. Oswald
CrystEngComm, 2014, 16, 5827 DOI:10.1039/C4CE00211C
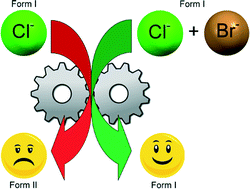
This paper describes a methodology for the modification of phase transition temperatures using (±)-4′-methylmethcathinone solid solutions as an exemplar. This method serves to show that by varying the composition of the halide ion one can systematically alter the temperatures at which phase transitions can occur in order to evade the possibility of interconversion between polymorphs during processing or storage of materials.
read at
http://pubs.rsc.org/en/Content/ArticleLanding/2014/CE/C4CE00211C?utm_medium=email&utm_campaign=pub-CE-vol-16-issue-26&utm_source=toc-alert#!divAbstract
DOI: 10.1039/C4CE00211C
Drug solid solutions – a method for tuning phase transformations
Amit Delori,a Pauline Maclure,a Rajni M. Bhardwaj,a Andrea Johnston,a Alastair J. Florence,a Oliver B. Sutcliffeb and Iain D. H. Oswald*a
Show Affiliations
*Corresponding authors
aStrathclyde Institute of Pharmacy and Biomedical Sciences (SIPBS), University of Strathclyde, 161 Cathedral Street, Glasgow, UK G4 0RE
E-mail: iain.oswald@strath.ac.uk;
Fax: +44 (0)141 552 2562 ;
Tel: +44 (0)141 548 2157
E-mail: iain.oswald@strath.ac.uk;
Fax: +44 (0)141 552 2562 ;
Tel: +44 (0)141 548 2157
bSchool of Science and the Environment, Manchester Metropolitan University, Chester Street, Manchester, UK M1 5GD
E-mail: o.sutcliffe@mmu.ac.uk;
Fax: +44 (0)161 2476840 ;
Tel: +44 (0)161 2471531
E-mail: o.sutcliffe@mmu.ac.uk;
Fax: +44 (0)161 2476840 ;
Tel: +44 (0)161 2471531
CrystEngComm, 2014,16, 5827-5831
DOI: 10.1039/C4CE00211C
Wednesday, 11 June 2014
Requirements for Process Validation of Biotech Active Pharmaceutical Ingredients (APIs)

Introduction
Process validation of Active Pharmaceuti-
cal Ingredients (APIs) is still one of the
most challenging topics for both pharma-
ceutical industry and regulatory authorities. It
is the clear expectation of regulatory authori-
ties, especially the FDA and EMEA, that pro-
duction processes, cleaning procedures, ana-
lytical methods, computer and utility systems
that have an impact on product quality and
purity are validated. Facilities and equipment
used in conjunction with production and testing
of APIs must be qualified. Thttp://www.eurotherm-danmark.dk/medic/pharma_doc/press_release/ispe_reprint_process_validation.pdf
Tuesday, 10 June 2014
Inhibiting Protein Aggregation
Inhibiting Protein Aggregation
 Conjugates of 8-hydroxyquinolines and cyclodextrins have been
developed that function both as antioxidants and as metal chelators
Conjugates of 8-hydroxyquinolines and cyclodextrins have been
developed that function both as antioxidants and as metal chelatorsRead more
The Biochemistry of Peppers – Part 2
The Biochemistry of Peppers – Part 2
 We are only able to taste sweet, bitter, sour, salty, and
umami – how do we taste hotness?
We are only able to taste sweet, bitter, sour, salty, and
umami – how do we taste hotness?Read more
Subscribe to:
Comments (Atom)