VENLAFAXINE PART 3/3

SEE
part 1………http://orgspectroscopyint.blogspot.in/2015/04/venlafaxine.html / http://newdrugapprovals.org/2015/04/09/venlafaxine-part-12/
SEE ALSO
PAPER
RSC Adv., 2014,4, 14468-14470
DOI: 10.1039/C4RA00840E
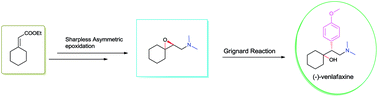
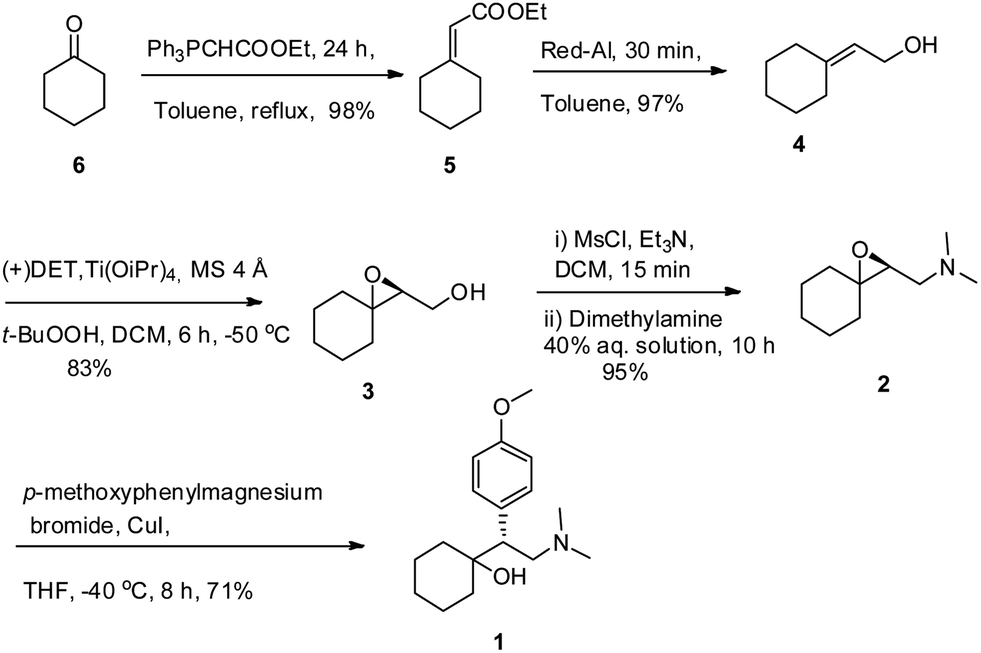
A protecting group free asymmetric total synthesis of (−)-venlafaxine is reported. The strategy employs Sharpless epoxidation and regio-selective epoxide ring opening by an in situgenerated Gilman reagent as key steps. This paper reports a 53% overall yield in 6 steps for total synthesis of (−)-venlafaxine.
…………………




























No comments:
Post a Comment