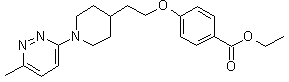
Pirodavir, R-77975
4 - [2 - [1 - (6-Methyl-3-pyridazinyl)-4-piperidinyl] ethoxy] benzoic acid ethyl ester
ethyl 4-{2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy}benzoate
ethyl 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]-ethoxy]benzoate
CAS REGISTRY NUMBER 124436-59-5
C21-H27-N3-O3
369.468
ANTIINFECTIVE THERAPY, Antiviral Drugs
Phase II
Pirodavir (R 77975) is the prototype of a novel class of broad-spectrum antipicornavirus compounds. Although its predecessor, R 61837, a substituted phenyl-pyridazinamine, was effective in inhibiting 80% of 100 serotypes tested (EC80) at concentrations above 32 micrograms/ml, pirodavir inhibits the same percentage of viruses at 0.064 micrograms/ml. Whereas R 61837 was active almost exclusively against rhinovirus serotypes of antiviral group B, pirodavir is broad spectrum in that it is highly active against both group A and group B rhinovirus serotypes.
Pirodavir is also effective in inhibiting 16 enteroviruses, with an EC80 of 1.3 micrograms/ml. Susceptible rhinovirus serotypes were rendered noninfectious by direct contact with the antiviral compound. Their infectivity was not restored by dilution of virus-drug complexes, but was regained by organic solvent extraction of the compound for most serotypes.
Neutralized viruses became stabilized to acid and heat, strongly suggesting a direct interaction of the compounds with viral capsid proteins. Mutants resistant to R 61837 (up to 85 times the MIC) were shown to bear some cross-resistance (up to 23 times the MIC) to the new compound, indicating that pirodavir also binds into the hydrophobic pocket beneath the canyon floor of rhinoviruses.
Pirodavir acts at an early stage of the viral replication cycle (up to 40 min after infection) and reduces the yield of selected rhinoviruses 1,000- to 100,000-fold in a single round of replication.
The mode of action appears to be serotype specific, since pirodavir was able to inhibit the adsorption of human rhinovirus 9 but not that of human rhinovirus 1A. Pirodavir is a novel capsid-binding antipicornavirus agent with potent in vitro activity against both group A and group B rhinovirus serotypes.
US 4992433
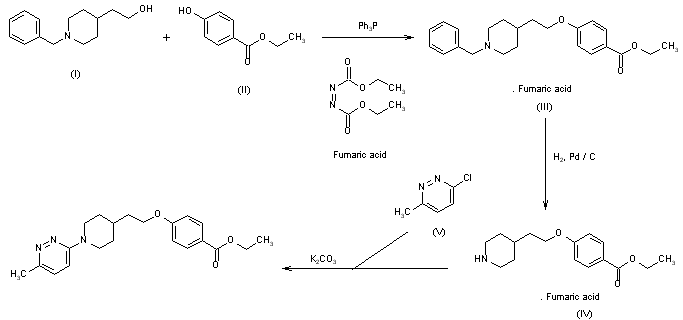
The condensation of 2-(1-benzylpiperidin-4-yl)ethanol (I) with 4-hydroxybenzoic acid ethyl ester (II) by means of triphenylphosphine and diazenedicarboxylic acid diethyl ester in THF gives 4-[2-(1-benzylpiperidin-4-yl)ethoxy]benzoic acid ethyl ester (III) as fumarate salt. This compound is debenzylated by hydrogenation with H2 over Pd/C in ethanol, yielding the free product (IV), which is finally condensed with 3-chloro-6-methylpyridazine (V) by means of K2CO3 in DMF.
Intermediate Name8-methoxy-2-methyl-4 (3H)-quinazolinone(I)
.......................................
B. Preparation of the Final Compounds EXAMPLE 25
A mixture of 10.4 parts of 3-chloro-6-methylpyridazine, 22.4 parts of ethyl 4-[2-(4-piperidinyl)ethoxy]benzoate butanedioate (1:1), 8.6 parts of sodium carbonate and 0.9 parts of N,N-dimethylformamide was stirred for 3 hours in an oil bath at .+-.150.degree. C. After cooling, water and dichloromethane were added and the layers were separated. The organic layer was dried, filtered and evaporated. The residue was purified by column chromatography over silica gel using a mixture of trichloromethane and ethanol (99:1 by volume) as eluent. The pure fractions were collected and the eluent was evaporated. The residue was crystallized from a mixture of 2,2'-oxybispropane and 2-propanone (75:25 by volume). The precipitated product was filtered off and dried, yielding 17 parts (56.8%) of ethyl 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]-ethoxy]benzoate; mp. 130.1.degree. C. (comp. 1).
.................
Journal of Medicinal Chemistry, 2003 , vol. 46, 15 p. 3181 - 3184

Scheme 1. Synthesis of Pirodavir (3) and Related Compounds
see mp and nmr data in supp file
| US2985657 * | Oct 12, 1959 | May 23, 1961 | Paul A J Janssen | 1-(aroylalkyl)-4-heterocyclylpiperazines |
| US4068383 * | Sep 30, 1976 | Jan 17, 1978 | Hoechstmass Balzer Gmbh & Co. | Tape measure reel |
| US4451476 * | Oct 17, 1983 | May 29, 1984 | Sterling Drug Inc. | Isoxazoles as antiviral agents |
| US4604127 * | May 15, 1985 | Aug 5, 1986 | Eli Lilly And Company | Herbicidal pyridazinylimidazolidinone compounds |
| EP0137242A2 * | Aug 20, 1984 | Apr 17, 1985 | Sterling Winthrop Inc. | (Substituted) Phenyl-aliphatic-isoxazoles useful as antiviral agents and preparation thereof |
| EP0156433A2 * | Mar 15, 1985 | Oct 2, 1985 | Janssen Pharmaceutica N.V. | Anti-virally active pyridazinamines |
| EP0211457A2 * | Jul 9, 1986 | Feb 25, 1987 | Janssen Pharmaceutica N.V. | Novel (4-substituted-piperazinyl)pyridazines |
| JPS5877866A * | Title not available |
read also
Antimicrobial Agents and Chemotherapy, 1995 , vol. 39, 2 p. 290 - 294

No comments:
Post a Comment