
- C26H20Cl2N4O4S
- mass: 555.432373 Da
read poster
http://www.cerep.fr/cerep/users/pages/news/Publications/123.pdf
BMS-587101 acts as a leukocyte function-associated antigen-1 (LFA-1) receptor antagonist. Ref: Synfacts. 2010; 8, 0865-0865.
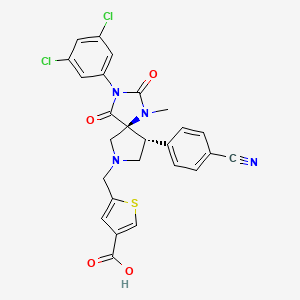
5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-ylmethyl]-thiophene-3-carboxylic Acid
3-Thiophenecarboxylic acid, 5-[[(5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl]methyl]- [ACD/Index Name]
5-{[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl]methyl}-3-thiophenecarboxylic acid [ACD/IUPAC Name]
5-{[(5S,9R)-9-(4-Cyanphenyl)-3-(3,5-dichlorphenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl]methyl}-3-thiophencarbonsäure [German] [ACD/IUPAC Name]
Acide 5-{[(5S,9R)-9-(4-cyanophényl)-3-(3,5-dichlorophényl)-1-méthyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl]méthyl}-3-thiophènecarboxylique [French] [ACD/IUPAC Name]
2IC
BMS-587101
BMS-688521
data
MS (ESI)m/z553 (M-H)-;
1H NMR(500 MHz, DMSO-d6)δ
8.17 (1 H, s), 7.62 (2 H, d,J=8.07 Hz), 7.44 (1 H, s), 7.27 (3 H, m), 6.64 (2 H, s),
4.11 (1 H, d,J=13.45 Hz), 3.96 (1 H, d,J=14.12 Hz), 3.88 (1 H, dd,J=11.76, 5.71 Hz), 3.43 (2 H, br. s.),
3.27 (1 H, br. s.), 3.23 (3 H, s), 3.06 (1 H, d,J=10.08 Hz);
Anal.(C26H20Cl2N4O4S)Calcd.: C,56.22; H,3.63;Cl, 12.77; N,10.09; S,5.77;.
Found: C,55.95; H,3.59;Cl, 12.54; N,10.01; S,5.79;
ee =99.26±0.00 % [Chiralcel OJ-R, 150 x 4.6 mm, 5 um particle size, MeOH: CH3CN: 0.2% aq.H3PO4 (30:30:40)];
[α]D=-6.324 (c = 8.967 mg/mL, CHCl3);
Interaction between leukocyte function-associated antigen-1 (LFA-1), expressed on the surface of cytokine-stimulated cells, and intercellular adhesion molecule (I-CAM), found on the surface of both leukocytes and endothelium, plays a key function in the intercellular immune response, causing T-cell adhesion and subsequent migration through the blood vessel wall to the inflamed area.(1)
Small molecules which inhibit the LFA-1/I-CAM interaction are targeted as potential drugs for the treatment of a variety of autoimmune and inflammatory diseases such as rheumatoid arthritis and psoriasis.(2, 3) The LFA-1 receptor antagonist, BMS-587101, 1,(4, 5) was selected for clinical development, and we required a synthesis that would reliably generate kilogram quantities of API. This paper details the identification and development of a synthesis which enabled the realization of this goal.
BMS-587101 inhibits the interaction between leukocyte function-associated antigen-1 (LFA-1) and the intercellular adhesion molecule (ICAM), thereby offering a potential treatment for various autoimmune and inflammatory diseases, such as rheumatoid arthritis and psoriasis. A four-step multikilogram route to BMS-587101 (22% overall yield ) from the commercial hydantoin B features an efficient dipolar cycloaddition of an azomethine ylide generated by reaction of glycine with hexamethylenetetramine (HMTA).
.............
paper


Org. Process Res. Dev., 2010, 14 (3), pp 553–561
DOI: 10.1021/op9003168
The process development and the kilogram-scale synthesis of BMS-587101 (1) are described. The synthesis features a [3 + 2] azomethine ylide cycloaddition to efficiently build the spirocyclic core in a diastereoselective fashion followed by a classical resolution which affords the desired enantiomer in >98% enantiomeric excess. The target was prepared in four steps in an overall yield of 22%.
Preparation of 5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-ylmethyl]-thiophene-3-carboxylic Acid (1) Directly from 6
To a solution of 6 (46.9 kg, 77.6 mol) and 1,2-propanediol (11.8 kg) in tetrahydrofuran (41.7 kg) and water (266.8 kg) was added cold (0−10 °C) potassium hydroxide solution (1 N, 244.5 kg) at 8−12 °C in 0.5 h. The resulting biphasic mixture was stirred at 8−12 °C for 18−24 h until the reaction was complete (<1% 6 remaining as monitored by HPLC). The reaction mixture was washed with n-heptane (385.7 kg). The pH was adjusted to 7.5 with addition of 1.5 M citric acid (22.9 kg). Isopropyl acetate (817.8 kg) was charged, and 1.5 M citric acid(aq) ( 22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of
22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of  207 L was attained. Heptane (117.8 kg) was charged, the slurry was cooled to 20 °C over 1.5 h and was subsequently wet milled until d90 < 60 μm. The slurry was held for >2 h and filtered. The cake was washed with a 1:1 isopropyl acetate/heptane solution (109.7 kg) isopropyl acetate and dried in vacuum at 35−40 °C to a constant weight. Acid 1 (39.6 kg, 91.5% yield and 99.33 HPLC area % purity) was obtained as a white and sandy crystalline solid.
207 L was attained. Heptane (117.8 kg) was charged, the slurry was cooled to 20 °C over 1.5 h and was subsequently wet milled until d90 < 60 μm. The slurry was held for >2 h and filtered. The cake was washed with a 1:1 isopropyl acetate/heptane solution (109.7 kg) isopropyl acetate and dried in vacuum at 35−40 °C to a constant weight. Acid 1 (39.6 kg, 91.5% yield and 99.33 HPLC area % purity) was obtained as a white and sandy crystalline solid.
 22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of
22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of  207 L was attained. Heptane (117.8 kg) was charged, the slurry was cooled to 20 °C over 1.5 h and was subsequently wet milled until d90 < 60 μm. The slurry was held for >2 h and filtered. The cake was washed with a 1:1 isopropyl acetate/heptane solution (109.7 kg) isopropyl acetate and dried in vacuum at 35−40 °C to a constant weight. Acid 1 (39.6 kg, 91.5% yield and 99.33 HPLC area % purity) was obtained as a white and sandy crystalline solid.
207 L was attained. Heptane (117.8 kg) was charged, the slurry was cooled to 20 °C over 1.5 h and was subsequently wet milled until d90 < 60 μm. The slurry was held for >2 h and filtered. The cake was washed with a 1:1 isopropyl acetate/heptane solution (109.7 kg) isopropyl acetate and dried in vacuum at 35−40 °C to a constant weight. Acid 1 (39.6 kg, 91.5% yield and 99.33 HPLC area % purity) was obtained as a white and sandy crystalline solid.
..............................
U.S. Patent 7,381,737 B2
IIIn:
Also provided are crystalline forms of solvates and salts of the substituted spiro-hydantoin compound (IIIn).
5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid.
EXAMPLES
The following examples illustrate embodiments of the inventive process, and are not intended to limit the scope of the claims. For ease of reference, the following abbreviations are used herein:
ABBREVIATIONS
- DMSO=dimethyl sulfoxide
- DTTA=(+)-Di-p-toluoyl-D-tartaric acid
Preparation 13-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione
Triethylamine (0.78 kg, 7.75 mol) was added in 15-30 minutes with stirring to a thin suspension of sarcosine ethylene hydrochloride (1.00 kg, 6.51 mol) in dichloromethane (6.00 L). After stirring at room temperature for 1.5-2.0 hours, the mixture was filtered to remove the resulting triethylamine hydrochloride salt. The salt cake was washed with dichloromethane (2.00 L). The filtrate was cooled to 0-5° C.
A solution of 3,5-dichlorophenyl isocyanate (1.47 kg, 7.81 mol) in dichloromethane was prepared at 20-25° C. The solution was added to the above cooled filtrate slowly in 30-60 minutes. The temperature was maintained below 10° C. during the addition. After the addition, the mixture was stirred at 20-25° C. for 12-14 hours. The completeness of the reaction was followed by HPLC. Upon reaction completion, TBME (16.00 L) was added in one portion. The resulting suspension was stirred at 20-25° C. for 2-3 hours and was then filtered. The filter cake was washed with TBME (4.50 L) and dried at maximum 40° C. to a constant weight. A suspension of the above filter cake in water (17.0 L, 10 L/kg input) was prepared and stirred at 20-25° C. for at least 16 hours. The suspension was filtered and the filter cake was washed with water (3×1.36 L) and dried at maximum 40° C. to a constant weight to a constant weight. 3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione (1.52 kg, 90%) was obtained as a white crystalline solid. mp=202-204° C. 1H NMR (DMSO-d6): 7.66 (1H, m), 7.51 (2H, m), 4.10 (2H, s), 3.35 (3H, s). 13C NMR (DMSO-d6): 8 Carbons (169.30, 155.00, 134.98, 134.15, 127.59, 125.30, 51.75, 29.79). Anal. Calcd for C10H8Cl2N2O2: C, 46.35; H, 3.11; N, 10.81; Cl, 27.36. Found: C, 46.43; H, 2.9; N, 10.73; Cl, 27.33.
Preparation 2(E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile
A mixture of 3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione (1.00 kg, 3.86 mol), 4-cyanobenzaldehyde (0.70 kg, 5.79 mol) and pyrrolidone (0.27 kg, 3.86 mmol) was refluxed in EtOH (13.00 L) for 20-24 hours at a temperature of 78° C. The completeness of the reaction was followed by HPLC. Upon reaction completion, the suspension was cooled to 65° C. and THF (4.33 L) was added in 5-10 minutes. The suspension was cooled to 20-25° C. in 3-4 hours and was then filtered. The filter cake was washed with EtOH (4×2.00 L) and dried at maximum 40° C. to a constant weight. (E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile (1.24 kg, 86%) was obtained as a fluffy, yellowish crystalline solid. mp=239-241° C. 1H NMR (DMSO-d6): 8.07 (2H, d, J=8.3 Hz), 7.86 (2H, d, J=8.4 Hz), 7.72 (1H, m), 7.59 (2H, m), 6.72 (1H, s), 3.35 (3H, s). 13C NMR (DMSO-d6): 14 Carbons (159.80, 151.48, 137.64, 133.83, 133.70, 131.80, 130.80, 130.68, 127.71, 125.51, 118.83, 114.48, 110.32, 26.72). Anal. Calcd for C18H11Cl2N3O2: C, 58.08; H, 2.97; N, 11.29; Cl, 19.05. Found: C, 58.14; H, 2.72; N, 11.14; Cl, 19.15.
Example 14-[(5S*,9R*)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile hydrochloride salt
A mixture of (E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile (1.00 kg, 2.69 mol), glycine (0.50 kg, 6.72 mol) and hexamethylenetetramine (0.28 kg, 2.02 mol) in 1-methyl-2-pyrrolidinone (5.00 L) and toluene (2.50 L) was heated at 140° C. for 7-8 hours. The completeness of the reaction was followed by HPLC. Upon reaction completion, the mixture was cooled to 40-50° C. and filtered. The filtered solid was washed with toluene (0.67 L). To the filtrate was added HCl (1M, 13.33 L, 13.33 mol). The resulting biphasic mixture was heated to 50-60° C. and was stirred for 10-15 minutes. The aqueous phase was separated and the organic phase was washed with HCl (1M, 1.67 L, 1.67 mol) at 60-80° C. The aqueous phases were combined and were stirred at 80° C. for 2 hours. The solution was cooled slowly in 3-4 hours to 20-25° C. with gentle stirring and seeding. Crystallization occurred and the resulting suspension was put aside at 20-25° C. for at least 16 hours with occasional stirring, cooled to 0-5° C. in 2 hours, stirred gently at 0-5° C. for 2 hours and then filtered. The filter cake was washed with ice water (2×2.50 L) and dried at maximum 40° C. to a constant weight. 4-[(5S*,9R*)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile hydrochloride salt (1.09 kg, 90%) was obtained as beige crystalline solid. mp=183-185° C. 1H NMR (DMSO-d6): 7.87(2H, d, J=8.1 Hz), 7.61 (1H, m), 7.40 (2H, d, J=8.1 Hz), 6.68 (2H, m), 4.17 (1H, m), 3.85 (2H, m), 3.76 (2H, m), 3.43 (3H, s), 3.24(2H, s). 13C NMR (DMSO-d6): 14 Carbons (170.84, 152.92, 137.35, 133.94, 132.87, 132.35, 128.01, 124.50, 118.12, 111.30, 71.42, 46.57, 45.11, 25.51). Anal. Calcd for C20H17Cl3N4O2+1.3 H2O: C, 50.51; H, 3.91; N, 11.79; Cl, 22.39. Found: C, 50.56; H, 3.86; N, 11.58; Cl, 21.98; KF, 5.12.
Example 2a4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile semi (+)-DTTA salt
To a suspension of 4-[(5S*,9R*)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile hydrochloric acid salt (1.00 kg, 2.21 mol) in dichloromethane (10.67 L) was added diispopropylethylamine (0.29 kg, 2.21 mol). The mixture was stirred to a clear solution, to which (+)-Di-p-toluoyl-D-tartaric acid (0.21 kg, 0.55 mol) was added. The resulting solution was warmed to 34-36° C. and seeded immediately. It was cooled to 20-25° C. in 1.5-2.0 hours. Crystallization occurred during cooling. TBME (2.75 L) was added in 0.5 hours. The suspension was stirred at 20-25° C. for 16 hours and then filtered. The filter cake was washed with dichloromethane/TBME (2/1, 1.00 L), TBME (1 L) and dried at maximum 35° C. to a constant weight. 4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile semi (+)-DTTA salt (0.47 kg, 35%) was obtained as a white crystalline solid. mp=175-177° C. 1H NMR (DMSO-d6): 7.86 (2H, d, J=8.1 Hz), 7.81 (2H, d, J=8.3 Hz), 7.61 (1H, m), 7.28 (2H, d, J=8.1 Hz), 7.22 (2H, 8.5 Hz), 6.68 (2H, m), 5.71 (1H, s), 3.81(1H, m), 3.50 (4H, m), 3.06 (3H, s), 2.34 (3H, s). 13C NMR (DMSO-d6): 24 Carbons (171.45, 169.40, 165.04, 152.88, 143.61, 138.99, 133.88, 133.08, 132.16, 129.26, 129.20, 128.76, 127.84, 126.99, 124.51, 118.25, 110.78, 72.81, 73.38, 48.15, 47.51, 46.30, 24.90, 21.14). Anal. Calcd for C30H25Cl2N4O6+0.5 H2O: C, 58.40; H, 4.17; N, 9.08; Cl, 11.49. Found C, 58.58; H, 4.06; N, 8.94; Cl, 11.38; KF, 1.59.
Example 2b4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile semi (+)-DTTA salt
A mixture of (E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile (10.0 g, 26.9 mmol), glycine (5.06 g, 67.4 mmol), hexamethylenetetramine (2.82 g, 20.1 mmol) in 50 mL N-methylpyrrolidinone and 25 mL of toluene under nitrogen was heated to 138° C. for approximately 12 h. Next, 25 mL toluene and 25 mL H2O were added. The aqueous and nonaqueous layers were split, and the aqueous layer was washed with 25 mL of toluene, and the nonaqueous layers were combined to form a nonaqueous mixture. The nonaqueous mixture was heated to 45-50° C. and ethylene diamine (7.0 mL) was added. The nonaqueous mixture was stirred for 3 hours and then cooled to room temperature. Next, 50 mL H2O was added, followed by the addition of 10 mL brine. The next addition was 25 mL toluene, which was followed by the addition of 125 mL CH2Cl2. The bottom layer of the mixture was removed through a filter. Next, (+)-Di-p-toluoyl-D-tartaric acid (2.59 g, 6.7 mmol) was added and the mixture was stirred for 18 h to form a slurry. Slowly 40 mL of MTBE was added to the slurry. A wash solution containing 7 mL of MTBE and 11 mL of CH2Cl2 was prepared. Filter paper was wetted with 1 mL of the wash solution. The slurry was filtered and then the filtered to form a cake. The filter, the wash reaction flask, and the cake were washed with the remaining 16 mL of the wash solution. Next, the cake was washed with 10 mL MTBE. 4-[(5S, 9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile semi (+)-DTTA salt (4.0 g, 20% yield) was obtained as a white solid (98.7% HPLC AP and 98.3% ee).
Example 2c4-[(5S,9R)-3-3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4,4]non-9-yl]-benzonitrile semi (+)-DTTA salt
A mixture of (E)-4-((1-(3,5)-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile (40.0 g, 107.5 mmol), glycine (19.76 g, 263.2 mmol), hexamethylenetetramine (9.07 g, 64.7 mmol) in 200 mL N-methyl-2-pyrrolidinone and 100 mL of toluene was heated under nitrogen to 143° C. for approximately 5.5 h. Next, the mixture was cooled to 50° C. and a solution of 25 mL of ethylenediamine in 200 mL of tetrahydrofuran was added. The mixture was maintained at a temperature of 50° C. for 30 minutes and then was cooled to room temperature. Next, 520 mL of 20 wt % NaCl aqueous solution was added. The aqueous and nonaqueous layers were separated. The nonaqueous layer was transferred to a vacuum distillation apparatus and solvent was distilled off until the temperature of the residue in the flask reached 58° C. at a pressure of 60 torr. Next, 360 mL of methylene chloride was added, followed by the additions of 20 mL of methanol and 2 mL of water. The next addition was (+)-Di-p-toluoyl-D-tartaric acid (10.38 g, 26.9 mmol), followed by 120 mL of methylene chloride and 0.200 g of seeds of 4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4,4]non-9-yl]-benzonitrile semi (+)-DTTA salt. A-slurry was formed and was stirred at room temperature for 24 hours. The slurry was filtered and the cake of crystals was washed with 200 mL of methylene chloride in two portions. The washed cake was then dried at 50° C. under vacuum for 24 hours. A total amount of 20.11 g (yield 31%) of 4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4,4]non-9-yl]-benzonitrile semi (+)-DTTA salt, which was of greater than 99.5% area percent purity, 98.4% potency and 99.2% ee was obtained after drying.
Example 35-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid methyl ester hydrochloride salt
To a suspension of 4-[(5S,9R)-3-(3,5-Dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-9-yl]-benzonitrile semi (+)-DTTA salt (7.50 kg, 12.30 mmol) and methyl 5-formylthiophene-3-carboxylate (2.2 kg, 13.10 mol) was added triethylamine (2.08 kg, 20.60 mol) at 20-25° C. The mixture was stirred to a clear solution, to which acetic acid (1.24 kg, 20.60 mol) was added. The resulting mixture was stirred at 20-25° C. for 1 hour and then cooled to 15° C. Solid sodium triacetoxyborohydride (1.31 kg, 6.17 mol) was added and the reaction mixture was stirred for 0.5 hours. The addition of sodium triacetoxyborohydride was repeated three more times. At the end, a total of 5.22 kg (24.7 mol) sodium triacetoxyborohydride was added in 2 hours. The reaction mixture was stirred at 20-25° C. for 16 hours. The completeness of the reaction was followed by HPLC. Upon reaction completion, TBME (48.1 L) was added to the resulting jelly reaction mixture. The mixture was washed with saturated sodium hydrogen carbonate solution (60.0 L×3). The combined aqueous phase was extracted with TBME (48.1 L). All organic layers were combined, washed with brine (48.1 L) and concentrated in vacuum to a volume of 10.6 L. Isopropanol (192.3 L) was added to the residue and the resulting oil precipitates were dissolved upon warming up to 70-75° C. The solvent volume was reduced to 160.0 L by distillation at 70-75° C. Concentrated HCl (1.5 L) was added at 75° C. in 10 minutes followed by the addition of seed crystals. Crystallization occurred upon cooling to 20-25° C. in 16 hours. The mixture was filtered. The cake was washed with isopropanol (9.6 L×2) and dried at maximum 40° C. to a constant weight. 5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid methyl ester hydrochloride salt (6.57 kg, 88.0%) was obtained as white crystalline solid. mp=204-207° C. 1H NMR (CDCl3): 14.22 (1H, b), 8.18 (1H, d, J=0.9 Hz), 7.86 (1H, m), 7.67 (2H, d, J=8.1 Hz), 7.24 (1H, m), 7.23 (2H, d, J=8.1 Hz), 6.67 (2H, m), 4.76 (2H, m), 4.46 (1H, m), 4.16 (1H, m), 4.02 (2H, m), 3.86 (3H, s), 3.75 (1H, m), 3.38 (3H, s). 13C NMR (CDCl3): 18 Carbons (171.24, 162.32, 152.98, 136.05, 135.27, 134.03, 132.83, 131.94, 130.46, 128.85, 128.56, 123.92, 117.52, 113.43, 71.13, 52.43, 52.22, 46.73). Anal. Calcd for C27H23Cl3N4O4S: C, 53.52; H, 3.83; N, 9.25; S, 5.29; Cl, 17.55. Found: C, 53.07; H, 3.69; N, 9.08; S, 5.23; Cl, 17.20.
Example 45-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid
To a solution of 5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid methyl ester hydrochloride salt (20.00 g, 33.00 mmol) and 1,2-propanediol (5.0 g) in tetrahydrofuran (200 mL) and water (100 mL) was added slowly potassium hydroxide solution (0.85M, 116 mL) at 8-12° C. in 0.5 hours. The resulting biphasic mixture was stirred at 8-12° C. for 20-27 hours until the reaction was complete. The reaction mixture was washed with n-heptane (200 mL). The pH was adjusted to 6.5 with addition of water (100 mL) and acetic acid (2.5 mL). Tetrahydrofuran was removed under reduced pressure at internal temperature <40° C. The pH was adjusted to 4.5 with addition of isopropyl acetate (400 mL) and acetic acid (11 mL). After 10 minutes of stirring, the aqueous layer was separated and was extracted with isopropylacetate (200 mL). The organic layers were combined, washed with water (100 mL) and concentrated under reduced pressure to a volume of 190 mL at bath temperature <40° C. Crystallization occurred during concentration. The crystal slurry was stirred at 20-25° C. for 16 hours and was then filtered. The cake was washed with cold isopropylacetate (15 mL×3) and dried in vacuum at 35-40° C. to a constant weight.
5-[(5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-ylmethyl]-thiophene-3-carboxylic acid (14.35 g, 78.3%) was obtained as white and sandy crystalline solid.
mp=209-230° C. 1H NMR (Acetone-d6): 8.19 (1H, d, J=1.3 Hz), 7.76 (2H, d, J=8.4 Hz), 7.49 (2H, d, J=8.2 Hz), 7.43 (1H, d, J=1.0 Hz), 7.41 (1H, t, J=1.9 Hz), 6.87 (2H, d, J=1.9 Hz), 4.16 (1H, dd, J1=13.9 Hz J2=0.8 Hz), 4.10 (1H, dd, J1=11.7 Hz, J2=6.2 Hz), 3.99 (1H, d, J=14.0 Hz), 3.48(1H, d, J=10.6 Hz), 3.47 (1H, dd, J1=9.6 Hz, J2=6.2 Hz), 3.25 (3H, s), 3.24 (1H, dd, J1=9.6 Hz, J2=11.7 Hz), 3.01 (1H, d, J=11.3 Hz).
13C NMR (Acetone-d6): 22 Carbons (172.69, 163.7, 153.98, 144.55, 142.23, 135.26, 135.09, 134.41, 133.89, 132.96, 130.33, 128.27, 126.98, 125.18, 119.07, 112.44, 74.28, 59.09, 56.45, 54.33, 50.73, 25.75).
Anal. Calcd for C26H20Cl2N4O4S: C, 56.22; H, 3.62; N, 10.08; S, 5.77; Cl, 12.76. Found: C, 56.27; H, 3.20; N, 9.97; S, 5.65; Cl, 12.68.
................................
paper
J. Med. Chem. 2006, 49, 6946

LFA-1 (leukocyte function-associated antigen-1), is a member of the β2-integrin family and is expressed on all leukocytes. This letter describes the discovery and preliminary SAR of spirocyclic hydantoin based LFA-1 antagonists that culminated in the identification of analog 8 as a clinical candidate. We also report the first example of the efficacy of a small molecule LFA-1 antagonist in combination with CTLA-4Ig in an animal model of transplant rejection.
http://pubs.acs.org/doi/suppl/10.1021/jm0610806/suppl_file/jm0610806si20060913_101747.pdf synthesis as compd 8
says
a white solid: Anal.RP-HPLCtR= 3.09min (method D, purity 99%);
MS (ESI)m/z553 (M-H)-;
1H NMR(500 MHz, DMSO-d6)δ
8.17 (1 H, s), 7.62 (2 H, d,J=8.07 Hz), 7.44 (1 H, s), 7.27 (3 H, m), 6.64 (2 H, s),
4.11 (1 H, d,J=13.45 Hz), 3.96 (1 H, d,J=14.12 Hz), 3.88 (1 H, dd,J=11.76, 5.71 Hz), 3.43 (2 H, br. s.),
3.27 (1 H, br. s.), 3.23 (3 H, s), 3.06 (1 H, d,J=10.08 Hz);
Anal.(C26H20Cl2N4O4S)
Calcd.: C,56.22; H,3.63;Cl, 12.77; N,10.09; S,5.77;.
Found: C,55.95; H,3.59;Cl, 12.54; N,10.01; S,5.79;
ee =99.26±0.00 % [Chiralcel OJ-R, 150 x 4.6 mm, 5 um particle size, MeOH: CH3CN: 0.2% aq.H3PO4 (30:30:40)];
[α]D=-6.324 (c = 8.967 mg/mL, CHCl3);
......................
U.S. Patent 7,199,125 B2
.............................
.U.S. Patent 6,710,064 B2
.............
REFERENCES
- For a discussion on the inhibition of LFA-1/ICAM-1as an approach to treating autoimmune diseases see:Yusuf-Makagiansar, H.; Anderson, M. E.; Yakovleva, T. V.; Murray, J. S.; Siahaan, T. J. Medicinal Research Reviews 2002, 22, 146
- 2.For a discussion of therapeutic options for treatment of psoriasis, see:Gottlieb, A. B. J. Acad. Dermatol 2005, 53, S3Larson, R. S.; Davis, T.; Bologa, C.; Semenuk, G.; Vijayan, S.; Li, Y.; Oprea, T.; Chigaev, A.; Buranda, T.; Wagner, C. R.; Sklar, L. A.
- 3.For other small molecule LFA-1/ICAM-1 antagonists as potential drugs please see:(a) Pei, Z.; Xin, Z.; Liu, G.; Li, Y.; Reilly, E. B.; Lubbers, N. L.; Huth, J. R.; Link, J. T.; von Geldern, T. W.; Cox, B. F.; Leitza, S.; Gao, Y.; Marsh, K. C.; DeVries, P.; Okasinski, G. F. J. Med. Chem. 2001, 44, 2913(b) Liu, G.; Huth, J. R.; Olejniczak, E. T.; Mendoza, R.; DeVries, P.; Leitza, S.; Reilly, E. B.; Olasinski, G. F.; Fesik, S. W.; von Geldern, T. W. J. Med. Chem. 2001, 44, 1202(c) Wu, J.-P.; Emeigh, J.; Gao, D. A.; Goldberg, D. R.; Kuzmich, D.; Miao, C.; Potocki, I.; Qian, K. C.; Sorcek, R. J.; Jeanfavre, D. D.; Kishimoto, K.; Mainolfi, E. A.; Nabozny, G.; Peng, C.; Reilly, P.; Rothlein, R.; Sellati, R. H.; Woska, J. R.; Chen, S.; Gunn, J. A.; O’Brien, D.; Norris, S. H.; Kelly, T. A. J. Med. Chem. 2004, 47, 5356(d) Last-Barney, K.; Davidson, W.; Cardozo, M.; Frye, L. L.; Grygon, C. a.; Hopkins, J. L.; Jeanfavre, D. D.; Pav, S.; Qian, C.; Stevenson, J. M.; Tong, L.; Zindell, R.; Kelly, T. A. J. Am. Chem. Soc. 2001, 123, 5643(e) Wang, G. T.; Wang, S.; Gentles, R.; Sowin, T.; Leitza, S.; Reilly, E. B.; von Geldern, T. W. Bioorg. Med. Chem. Lett. 2005, 15, 195(f) Wattanasin, S.; Albert, R.; Ehrhardt, C.; Roche, D.; Savio, M.; Hommel, U.; Welzenbach, K.; Weitz-Schmidt, G. Bioorg. Med. Chem. Lett. 2003, 12, 499
- 4.The Discovery work towards this target compound BMS-587101 is described in:Potin, D.; Launay, M.; Monatlik, F.; Malabre, P.; Fabreguettes, M.; Fouquet, A.; Maillet, M.; Nicolai, E.; Dorgeret, L.; Chevallier, F.; Besse, D.; Dufort, M.; Caussade, F.; Ahmad, S. Z.; Stetsko, D. K.; Skala, S.; Davis, P. M.; Balimane, P.; Patel, K.; Yang, Z.; Marathe, P.; Postelneck, J.; Townsend, R. M.; Goldfarb, V.; Sheriff, S.; Einspahr, H.; Kish, K.; Malley, M. F.; DiMarco, J. D.; Gougoutas, J. Z.; Kadiyala, P.; Cheney, D. L.; Tejwani, R. W.; Murphy, D. K.; Mcintyre, K. W.; Yang, X.; Chao, S.; Leith, L.; Xiao, Z.; Mathur, A.; Chen, B.-C.; Wu, D.-R.; Traeger, S. C.; McKinnon, M.; Barrish, J. C.; Robl, J. A.; Iwanowicz, E. J.; Suchard, S. J.; Dhar, M. T. G. J. Med. Chem. 2006, 49, 6946
- 5.For additional information related to this compound see:(a) Chen, B.-C.; DelMonte, A. J.; Dhar, T. G. M.; Fan, Y.; Gougoutas, J. Z.; Malley, M. F.; McLeod, D. D.; Waltermire, R.; Wei, C. Crystalline Forms and Process for Preparing Spiro-Hydantoin Compounds. (Bristol-Myers Squibb). U.S. Patent 7,381,737 B2 .(b) Dhar, T. G. M.; Potin, D.; Maillet, M.; Launay, M.; Nicolai, E.; Iwanowicz, E. Spiro-cyclic compounds useful as anti-inflammatory agents. Bristol-Myers Squibb and Cerep). U.S. Patent 7,199,125 B2.(c) Launay, M.; Potin, D.; Maillet, M.; Nicolai, E.; Dhar, T. G. M.; Iwanowicz, E. Hydantoin compounds useful as anti-inflammatory agents. (Bristol-Myers Squibb).U.S. Patent 6,710,064 B2.For the radiolabelled synthesis of BMS-587101 see:Tran, S. B.; Maxwell, B. D.; Chen, S.-Y.; Bonacorsi, S. J.; Leith, L.; Ogan, M.; Rinehart, J. K.; Balasubramanian, B. J. Labelled Compd. Radiopharm. 2009, 52, 236
10-31-2008
|
CRYSTALLINE FORMS AND PROCESS FOR PREPARING SPIRO-HYDANTOIN COMPOUNDS
| |
6-4-2008
|
Crystalline forms and process for preparing spiro-hydantoin compounds
| |
3-7-2007
|
Pyridyl-substituted spiro-hydantoin compounds and use thereof
| |
7-19-2006
|
Spiro-hydantoin compounds useful as anti-inflammatory agents
| |
6-30-2006
|
Pyridyl-substituted spiro-hydantoin crystalline forms and process
| |
12-21-2005
|
Spiro-hydantoin compounds useful as anti-inflammatory agents
|
| US8710058 * | Dec 4, 2009 | Apr 29, 2014 | Merck Patent Gmbh | Polymorphic forms of 3-(1-{3-[5-(1-methyl-piperidin-4-ylmethoxy)-pyrimidin-2-yl]-benzyl}-6-oxo-1,6-dihydro-pyridazin-3-yl)-benzonitrile hydrochloride salt and processes of manufacturing thereof |
| US20110269767 * | Dec 4, 2009 | Nov 3, 2011 | Merck Patent Gesellschaft Mit Beschrankter Haftung | Novel Polymorphic Forms of 3-(1--6-oxo-1,6-dihydro-pyridazin-3-yl)-benzonitrile Hydrochloride Salt and Processes of Manufacturing Thereof |








No comments:
Post a Comment