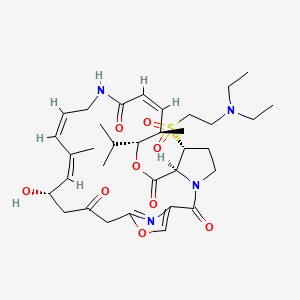
Dalfopristin
Dalfopristin;Dalfopristin Mesylate;(3R,4R,5E,10E,12E,14S,26R,26aS)-26-[[2-(DiethylaMino)ethyl]sulfonyl]-8,9,14,15,24,25,26,26a-octahydro-14-hydroxy-4,12-diMethyl-3-(1-Methylethyl)-3H-21,18-nitrilo-1H,22H-pyrrolo[2,1-c][1,8,4,19]dioxadiazacyclotetracosine-1,7,16,22(4H,17H)-tetr
Rhone-Poulenc Sante .....LINK
- Dalfopristin
- Dalfopristina
- Dalfopristina [INN-Spanish]
- Dalfopristine
- Dalfopristine [INN-French]
- Dalfopristinum
- Dalfopristinum [INN-Latin]
- RP 54476
- UNII-R9M4FJE48E
| |
|
Brief background information
| SALT | ATC | FORMULA | MM | CAS |
|---|---|---|---|---|
| - | J01FG02 | C 34 H 50 N 4 O 9 S | 690.86 g / mol | 112362-50-2 |
Application
- antibiotic (used for bacteremia caused by the vancomycin-resistant Enterococcus faecium )
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (3R,4R,5E,10E,12E,14S,26R,26aS)-26-[[2-(diethylamino)ethyl]sulfonyl]-8,9,14,15,24,25,26,26a- octahydro-14-hydroxy-3-isopropyl-4,12-dimethyl-3H-21,18-nitrilo-1H,22H-pyrrolo[2,1-c][1,8,4,19]-dioxadiazacyclotetracosine-1,7,16,22(4H,17H)-tetrone | |
| CLINICAL DATA | |
| AHFS/DRUGS.COM | International Drug Names |
| MEDLINEPLUS | a603007 |
| LEGAL STATUS | |
| PHARMACOKINETIC DATA | |
| HALF-LIFE | 1 hour |
| IDENTIFIERS | |
| CAS NUMBER | 112362-50-2 |
| ATC CODE | None |
| PUBCHEM | CID 6435782 |
| DRUGBANK | DB01764 |
| CHEMICAL DATA | |
| FORMULA | C34H50N4O9S |
| MOL. MASS | 690.85 g/mol |
Dalfopristin is a semi-synthetic streptogramin antibiotic analogue of ostreogyrcin A (virginiamycin M, pristinamycin IIA, streptogramin A).[1] The combination quinupristin/dalfopristin (marketed under the trade name Synercid) was brought to the market by Rhone-Poulenc Rorer Pharmaceuticals in 1999.[2] Synercid (weight-to-weight ratio of 30% quinupristin to 70% dalfopristin) is used to treatinfections by staphylococci and by vancomycin-resistant Enterococcus faecium.[3]
Synthesis
Through the addition of diethylaminoethylthiol to the 2-pyrroline group and oxidation of the sulfate of ostreogrycin A, a structurally more hydrophobic compound is formed. This hydrophobic compound contains a readily ionizable group that is available for salt formation.[1]
Large Scale Preparation
Dalfopristin is synthesized from pristinamycine IIa through achieving a stereoselective Michael-type addition of 2-diethylaminoethanethiol on the conjugated double bond of the dehydroproline ring [4] . The first method found was using sodium periodate associated with ruthenium dioxide to directly oxidize the sulfur derivative into a sulfone. However, using hydrogen peroxidewith sodium tungstate in a 2-phase medium produces an improved yield, and is therefore the method of choice for large scale production.
The production of the dalfopristin portion of quinupristin/dalfopristin is achieved through purifying cocrystallization of the quinupristin and dalfopristin from acetone solutions.[4]
Physical Characteristics (as mesylate salt)
| Appearance | White to yellow solid |
| Physical State | Solid |
| Solubility | Soluble in ethanol, methanol, DMSO, DMF, and water (0.072 mg/ml) |
| Storage | -20°C |
| Boiling Point | 940.5°C at 760 mmHg |
| Melting Point | 150°C |
| Density | 1.27 g/cm^3 |
| Refractive Index | n20D 1.58 |
| pK Values | pKa: 13.18 (Predicted), pKb: 8.97 (Predicted) |
Antimicrobial Activity
Alone, both dalfopristin and quinupristin have modest in vitro bacteriostatic activity. However, 8-16 times higher in vitro bactericidal activity is seen against many gram-positive bacteria when the two streptogramins are combined [5] . While quinupristin/dalfopristin is effective against staphylococci and vancomycin-resistant Enterococcus faecium, in vitro studies have not demonstrated bactericidal activity against all strains and species of common gram-positive bacteria.
Mechanism of Action
Both dalfopristin and quinupristin bind to sites located on the 50S subunit of the ribosome. Initial dalfopristin binding results in a conformational change of the ribosome, allowing for increased binding by quinupristin.[5] A stable drug-ribosome complex is created when the two drugs are used together. This complex inhibits protein synthesis through prevention of peptide-chain formation and blocking the extrusion of newly formed peptide chains. In many cases, this leads to bacterial cell death.
Mechanism of Resistance
Streptogramin resistance is mediated through enzymatic drug inactivation, efflux or active transport of drug out of the cell, and most commonly, conformational alterations in ribosomal target binding sites.[5] Enzymatic drug inactivation may occur in staphylococcal and enterococcal species through production of dalfopristin-inactivating acetyltransferase or quinupristin-inactivating hydrolase. Efflux or active transport of the drug may occur in coagulase-negative staphylococci and Enterococcus faecium. Constitutive ribosome modification has been seen in staphylococci with resistance seen in quinupristin only.
While resistance to dalfopristin may be conferred via a single point of mutation, quinupristin/dalfopristin offers the benefit of requiring multiple points of mutation targeting both dalfopristin and quinupristin components to confer drug resistance.[5] Comparatively, only 2-5% of staphylococcal isolates collected in France show resistance to a related streptogramin, pristinamycin, in over 35 years of use.
Drug Interactions
Both dalfopristin and quinupristin are extensively hepatically metabolized, excreted from the feces, and serve as an inhibitor of cytochrome P450 (CYP) 3A4 enzyme pathway.[5]Caution should be taken with concommitent use with drugs metabolized by the CYP3A4 pathway. Concomitant use of quinupristin/dalfopristin with cyclosporine for 2–5 days has shown to result in a two-fold increase in cyclosporine levels.
No adverse effects have been seen in patients with hepatic impairment and no recommendations by the manufacturer have been made for dose reduction ofquinupristin/dalfopristin in this patient population.
Commercialization
While little information is available regarding the regulatory and commercialization history of Dalfopristin alone, Synercid (quinupristin/dalfopristin), made by Rhone-Poulenc Rorer Pharmaceuticals, was approved in 1999 as an IV injectable for the treatment of vancomycin resistant Enterococcus faecium and complicated skin and skin structure infections.[2]Dalfopristin can be purchased alone on the internet from various chemical manufacturers as a mesylate salt.
Synthesis pathway
US 4668669
OR
- EXAMPLE 4
- By proceeding in a similar manner to that described in subs. Ple 1, but starting from 5.5 g of (2-dimethylamino ethyl) thio-26 pristinaffycine II B, of 0.67 cm3 trifluoroacetic acid 1.8 g of meta-chloroperbenzoic acid and after purification by "flash" chromatography [eluent: chloroform-methanol (90:10 by volume)], collecting fractions of 30 cm3 and concentration to dryness fractions 23-40 under reduced pressure (2.7 kPa) at 30 ° C, 0.4 g of (2-dimethylamino ethyl) sulfinyl-26 pristinamycin II B (isomer A 2 70% 1 15% A isomer, isomer B 1 7%, isomer B 28%) as a yellow powder melting at 150 ° C.
- The (2-dimethylamino ethyl) thio pristinamycin II B-26 can be prepared as follows:
- By proceeding in a similar manner to that described in Example 3, but using 2.7 g of pristinamycin II A and 0.58 g of dimethylamino-ethanethiol and 2 after purification by "flash" chromatography [eluent: chloroform -methanol (90:10 by volume)] and concentration to dryness fractions 11-17 under reduced pressure (2.7 kPa) at 30 ° C, 1.1 g of (2-dimethylamino ethyl) thio-26 pristinamycin II B as a yellow powder melting at 100 ° C.
- NMR spectrum:
- 2.35 (s, 6H:-N (CH 3) 2)
- 2.80 (m, 4H:-S-CH 2 CH 2 - <N)
- 3 40 (ddd, 1H: - H 26)
- 4.75 (d, 1 H, H-27)
- 8.10 (s, 1 H - H 20)
Trade Names
| COUNTRY | TRADE NAME | MANUFACTURER |
|---|---|---|
| Germany | Sinertsid | Aventis Pharma |
| United Kingdom | - "- | Aventis |
| Italy | - "- | Aventis |
| USA | - "- | Aventis |
| Ukraine | No | No |
Formulations
- injection of 180 mg / vial, 420 mg / vial
Links
- US 4,668,669 (Rhône-Poulenc Sante; 26.5.1987; F-prior. 11.1.1985).
- US 4,798,827 (Rhône-Poulenc Sante; 17.1.1989; F-prior. 22.5.1986).
- GB 2206879 (Rhône-Poulenc Rorer; appl. 7/7/1987; GB -prior. 18/1/1989).
References
- Dalfopristin (as mesylate) (CAS 112362-50-2)
- http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/50747_Synercid.cfm
- Allington DR, Rivey MP (2001). "Quinupristin/dalfopristin: a therapeutic review". Clin Ther 23 (1): 24–44. doi:10.1016/S0149-2918(01)80028-X. PMID 11219478.
- Barriere, J.C.; Berthaud, N.; Beyer, D.; Dutka-Malen, S.; Paris, J.M.; Desnottes, J.F. (April 1998). "Recent Developments in Streptogramin Research". Current Pharmaceutical Design 4 (2): 155–190. PMID 10197038. Retrieved 24 November 2013.
- Allington, Douglas R.; Rivey, Michael P. (January 2001). "Quinupristin/Dalfopristin: A Therapeutic Review". Clinical Therapeutics 23 (1): 1–21. doi:10.1016/S0149-2918(01)80028-X. PMID 11219478.
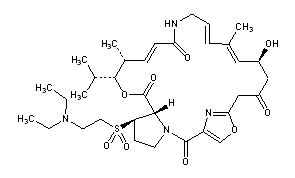
Title: Dalfopristin
CAS Registry Number: 112362-50-2
CAS Name: (26R,27S)-26-[[2-(Diethylamino)ethyl]sulfonyl]-26,27-dihydrovirginiamycin M1
Additional Names: 26-(2-diethylaminoethyl)sulfonylpristinamycin IIB
Manufacturers' Codes: RP-54476
Molecular Formula: C34H50N4O9S
Molecular Weight: 690.85
Percent Composition: C 59.11%, H 7.29%, N 8.11%, O 20.84%, S 4.64%
Literature References: Semisynthetic polyunsaturated macrolactone type II streptogramin, q.v. Prepn: J.-C. Barriere et al., EP191662; eidem, US 4668669 (1986, 1987 both to Rhone-Poulenc). In vitro activity: H. C. Neu et al., J. Antimicrob. Chemother. 30,Suppl. A, 83 (1992). HPLC determn in plasma: A. Le Liboux et al., J. Chromatogr. B 708, 161 (1998)
Properties: White solid, mp ~150°.
Melting point: mp ~150°
Derivative Type: Mixture with quinupristin
CAS Registry Number: 126602-89-9
Manufacturers' Codes: RP-59500
Trademarks: Synercid (Rh>e-Poulenc)
Literature References: Semisynthetic streptogramin comprised of two synergistic components in a defined 70:30 percent w/w mixture of dalfopristin and quinupristin, q.v., mesylate salts. HPLC determn for quality control: B. Vasselle et al., J. Pharm. Biomed. Anal. 19, 641 (1999). In vitro activity in comparison with pristinamycin, q.v.: A. Lozniewski et al., Pathol. Biol. 48, 463 (2000). Clinical trial in vancomycin resistant Enterococcus faecium (VREF) infection: R. C. Moellering et al., J. Antimicrob. Chemother. 44, 251 (1999); in skin infections: R. L. Nichols et al., ibid. 263. Review: B. Pavan, Curr. Opin. Invest. Drugs 1, 173-180 (2000).
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics).
| EP0252720A2 * | Jul 7, 1987 | Jan 13, 1988 | MAY & BAKER LIMITED | Pristinamycin process |
| EP0298177A1 * | Jul 7, 1987 | Jan 11, 1989 | Rhone-Poulenc Sante | Process for preparing pristinamycine IIB derivatives |
| US4866172 * | Apr 12, 1988 | Sep 12, 1989 | May & Baker Limited | Pristinamycin process |
| WO1992001693A1 * | Jul 15, 1991 | Jan 17, 1992 | Rhone Poulenc Rorer Sa | Method for the preparation of sulphinyl pristinamycin ii¿b? |



No comments:
Post a Comment