OR
Encenicline (EVP-6124, MT-4666)
EVP-6124 , MT-4666, α7-nAChR agonist, UNII-5FI5376A0X
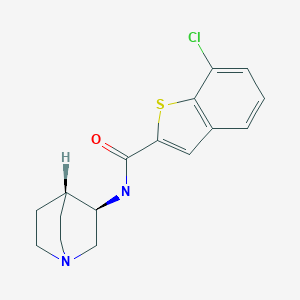
Chemical Name: (R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide
Therapy Type: Small Molecule
Target Type: Cholinergic System
Chemical Name: (R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide
Therapy Type: Small Molecule
Target Type: Cholinergic System
CAS : 550999-75-2
- C16 H17 Cl N2 O S
- Benzo[b]thiophene-2-carboxamide, N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-7-chloro-
- (R)-7-Chloro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide; EVP 6124
Condition(s): Alzheimer’s Disease, Schizophrenia
U.S. FDA Status: Alzheimer’s Disease (Phase 3), Schizophrenia (Phase 3)
Status in Select Countries: Investigational in Japan
Company: FORUM Pharmaceuticals Inc. (was EnVivo Pharmaceuticals), Mitsubishi Tanabe Pharma
Approved for: None AS ON SEPT 2014
U.S. FDA Status: Alzheimer’s Disease (Phase 3), Schizophrenia (Phase 3)
Status in Select Countries: Investigational in Japan
Company: FORUM Pharmaceuticals Inc. (was EnVivo Pharmaceuticals), Mitsubishi Tanabe Pharma
Approved for: None AS ON SEPT 2014
CAS 550999-74-1
Benzo[b]thiophene-2-carboxamide, N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-7-chloro-, monohydrochloride
(R)-7-Chloro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide hydrochloride
Mitsubishi Tanabe Pharma ..Encenicline-hydrochloride (EVP-6124) for Alzheimer’s disease by partner EnVivo Pharmaceuticals. Mitsubishi Tanabe has licensed EVP-6124 from EnVivo and is currently developing the drug under the code MT-4666.
The drug is a new alpha-7 potentiator intended to improve cognition in patients affected with Alzheimer’s disease. The drug is being tested in Phase III COGNITIV clinical trials in two categories: COGNITIV AD in patients with Alzheimer’s disease and COGNITIV CIAS in patients with cognitive impairment associated with schizophrenia.
Alzheimer’s disease affects five million people in the U.S. alone, or one in eight Americans over the age of 65. The disease is the sixth-leading cause of death in the country, with the number of affected patients expected to balloon to nearly triple by 2030. Alzheimer’s disease is a complex neurodegenerative disease that eventually leads to cellular loss and dysfunction in the brain resulting in decline of language skills and reasoning among others.
Phase III of COGNITIV AD clinical trial program involves about 1,600 patients with mild to moderate AD and who are presently receiving stable treatment with or have undergone previous acetylcholinesterase inhibitor treatment. The trials will be placebo-controlled, double-blind, and randomized. Patients in the trial will be randomized to receive either one of two doses of MT-4666 once daily against a placebo to assess safety and efficacy of the drug.
In the news release recently launched by EnVivo, CEO and president Deborah Dunsire said, “We are pleased to advance encenicline into Phase 3 clinical development in Alzheimer’s disease, a significant milestone for our company and promising step forward for patients who desperately need new therapies…Prior clinical studies of encenicline have demonstrated clinically significant improvements in cognitive function in patients with Alzheimer’s disease. For the millions of patients living with AD, we believe encenicline has the potential to make a meaningful difference.”
Encenicline hydrochloride is a partial, selective agonist of the α-7 nicotinic acetylcholine receptor (α7-nAChR). It is being developed for the treatment of cognitive deficits in schizophrenia and Alzheimer’s disease. Cholinergic function declines in Alzheimer’s, and currently approved acetylcholinesterase inhibitor therapies modestly improve cognitive deficits in patients with AD by way of boosting cholinergic transmission. The rationale of selective α7-nAChR agonists is that they will enhance cognition without causing side effects associated with overactivation of other nAChRs such as α4β2, or muscarinic AChRs. In rats, encenicline penetrates the blood-brain barrier and improves memory performance by potentiating the acetylcholine response. Encenicline has been reported to act as a co-agonist with acetylcholine. It sensitizes the α-7 nACh receptor to its natural ligand and renders sub-efficacious doses of AChEI drugs effective in restoring memory function in an object recognition task (Prickaerts et al., 2012).
This compound was originally developed at Bayer Healthcare and then licensed to Envivo Pharmaceuticals, which subsequently licensed development in Asia to Mitsubishi Tanabe Pharma Corporation. Envivo then changed its name to FORUM Pharmaceuticals Inc.
Encenicline is being tested in Alzheimer’s disease and schizophrenia. In Alzheimer’s, an ascending-dose Phase 1/2 study showed 0.1 to 1 mg/day of EVP-6124 to be safe and well-tolerated when given to 49 people with mild to moderate AD for 28 days. No serious side effects were reported. Secondary efficacy endpoints suggested that EVP-6124 given in addition to therapy with the acetyl cholinesterase inhibitors donepezil or rivastigmine appeared to improve attention, verbal fluency, and executive function as measured on tests in the CogState or NTB batteries (see conference news story). This study has posted results on clinicaltrials.gov.
A 24-week Phase 2 trial conducted in 409 people with mild to moderate Alzheimer’s disease in the United States and Eastern Europe compared 0.3, 1, and 2 mg of EVP-6124 per day to placebo, measuring cognition with ADAS-Cog as the primary outcome plus cognitive, functional, and psychiaric secondary outcomes. EVP 6124 was given as adjunct therapy to donepezil or rivastigmine. This trial was reported to have met its primary and most secondary endpoints, showing that people on the highest dose improved over baseline. EVP-6124 dose-dependently improved measures of attention, verbal and language fluency, and executive function. In this trial, all treatment groups initially improved, possibly due to a placebo effect, but by 12 weeks the groups separated and the placebo and low-dose groups declined (see conference news story). EVP-6124 was well-tolerated.
Mitsubishi Tanabe Pharma Corporation is conducting a Phase 2 trial for the treatment of Alzheimer’s disease in Japan.
In October 2013, two international Phase 3 trials began enrolling what are to be 790 patients in each trial with mild to moderate Alzheimer’s who are already taking an acetylcholinesterase inhibitor. The trials will compare two fixed, undisclosed add-on doses of EVP-6124 to placebo, all given as once-daily tablets for six months, for cognitive benefit as measured by the ADAS-Cog, clinical benefit as measured by the Clinical Dementia Rating Sum of Boxes (CDR-SB), as well as for safety and tolerability. Called COGNITIV AD, this Phase 3 program is is set to run through 2016.
For schizophrenia, a Phase 1 study comparing 0.3 and 1 mg/day of EVP-6124 to placebo in 28 people with the disease gave preliminary evidence for the compound’s safety, tolerability, and pharmacokinetics in this population. In addition, the compound yielded signals of bioactivity in the brain by way of EEG tests of evoked potentials, a measure of sensory gating affected in this disease. See study results on clinicaltrials.gov.
A subsequent 12-week Phase 2 trial compared 0.3 and 1 mg/day of EVP-6124 to placebo in 317 people with schizophrenia and measured safety and the compound’s efficacy on cognitive function. As presented at the American College of Neuropsychopharmacology meeting held in Hawaii December 2011, EVP 6124 met its primary endpoint of improvement on the CogState overall cognition index. The study also met secondary endpoints, showing improvement in clinical function as assessed by the Schizophrenia Cognition Rating Scale, and a decrease in negative symptoms (See company press release).
Two six-month, 700-patient Phase 3 studies, plus a six-month extention study, are ongoing. For all clinical trials of encenicline, see clincialtrials.gov.
Synthesis (hereinafter, the compound of Reference Example 25) carboxamide hydrochloride (Reference Example 25) (R) -7 – chloro-N-(quinuclidin-3 – – yl) benzo [b] thiophene-2:
[First Step]
Synthesis of carboxamide (R) -7 – chloro-N-(quinuclidin-3 – – yl) benzo [b] thiophene-2:
[First Step]
Synthesis of carboxamide (R) -7 – chloro-N-(quinuclidin-3 – – yl) benzo [b] thiophene-2:
-N, N, N ‘, N’-tetra-7 – chloro-1 – benzothiophene -2 – – o-(yl benzotriazol-1) chloroform solution (210mg, 1.0mmol) of carboxylic acid in (10mL) was added (0.70mL, 4.0mmol) and (570mg, 1.5mmol), diisopropylethylamine methyl hexafluorophosphate, (R) – (200mg, 1.0mmol) amine hydrochloride – quinuclidine-3 was added, and the mixture was stirred at room temperature. 16 hours later, was added distilled water, 1.0N sodium hydroxide solution, and extracted with chloroform. Was washed with saturated brine and the organic layer was concentrated and then dried over anhydrous sodium sulfate. (Fuji Silysia Chemical amine silica gel DM1020, chloroform alone – chloroform / methanol = 90/10) on silica gel column chromatography of the crude product obtained was purified by the title compound; was obtained as a white solid (170mg 53%).
1 H-NMR (400MHz, DMSO-d 6)
δ :1.22-1 .38 (1H, m) ,1.53-1 .62 (2H, m) ,1.75-1 .82 (2H, m) ,2.63-2 .73 (4H , m) ,2.84-2 .94 (1H, m) ,3.07-3 .18 (1H, m) ,3.90-4 .00 (1H, m), 7.49 (1H, dd , J = 7.6,8.0 Hz), 7.59 (1H, d, J = 7.6Hz), 7.96 (1H, d, J = 8.0Hz), 8.31 (1H, s) ,8.62-8 .66 (1H, m).
MS (ESI): 321 [M + H] +
1 H-NMR (400MHz, DMSO-d 6)
δ :1.22-1 .38 (1H, m) ,1.53-1 .62 (2H, m) ,1.75-1 .82 (2H, m) ,2.63-2 .73 (4H , m) ,2.84-2 .94 (1H, m) ,3.07-3 .18 (1H, m) ,3.90-4 .00 (1H, m), 7.49 (1H, dd , J = 7.6,8.0 Hz), 7.59 (1H, d, J = 7.6Hz), 7.96 (1H, d, J = 8.0Hz), 8.31 (1H, s) ,8.62-8 .66 (1H, m).
MS (ESI): 321 [M + H] +
[Second Step]
Synthesis of the compound of Reference Example 25:
Synthesis of the compound of Reference Example 25:
Ethyl acetate solution – solution of hydrogen chloride in ethyl acetate (170mg, 0.53mmol) of the (2.0mL) carboxamide – (R) -7 – chloro-N-(quinuclidin-3 – yl) benzo [b] thiophene-2 was added (4.0M, 0.20mL, 0.80mmol), and the mixture was stirred at room temperature. 10 minutes later, by which is filtered off and the resulting solid was washed with ethyl acetate and hexane, and dried, the compound of Reference Example 25; was obtained as a white solid (170mg 90%).
1 H-NMR (400MHz, DMSO-d 6)
δ :1.70-1 .78 (1H, m) ,1.86-1 .94 (2H, m) ,2.10-2 .19 (2H, m) ,3.18-3 .35 (5H , m) ,3.63-3 .72 (1H, m) ,4.27-4 .36 (1H, m), 7.50 (1H, d, J = 7.6,8.0 Hz), 7 .61 (1H, d, J = 7.6Hz), 7.98 (1H, d, J = 8.0Hz), 8.38 (1H, s) ,9.07-9 .10 (1H, m) ,9.80-9 .85 (1H, m).
MS (ESI): 321 [M + H] +
1 H-NMR (400MHz, DMSO-d 6)
δ :1.70-1 .78 (1H, m) ,1.86-1 .94 (2H, m) ,2.10-2 .19 (2H, m) ,3.18-3 .35 (5H , m) ,3.63-3 .72 (1H, m) ,4.27-4 .36 (1H, m), 7.50 (1H, d, J = 7.6,8.0 Hz), 7 .61 (1H, d, J = 7.6Hz), 7.98 (1H, d, J = 8.0Hz), 8.38 (1H, s) ,9.07-9 .10 (1H, m) ,9.80-9 .85 (1H, m).
MS (ESI): 321 [M + H] +
………………………………….
Example 69
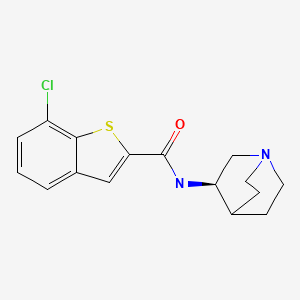
N-[(3 R) – 1 – azabicyclo [2.2.2] oct-3-y 1]-7-chloro-1-benzothiophene-2-carboxamide hydrochloride DESIRED
x HCI
176.2 mg (0.83 mmol) of 7-chloro-l-benzothiophene-2-carboxylic acid, 150 mg (0.75 mmol)
R-3-Aminochinuklidin dihydrochloride, 343.7 mg (0.90 mmol) of HATU, 350.5 mg
(2.71 mmol) of N, N-diisopropylethylamine and 3.0 ml of DMF are reacted according to the general working procedure (variant B). The reaction mixture is purified by preparative HPLC. The product will be in a mixture of 4 M HCl solution in dioxane and methanol, and then concentrated. This gives 175.2 mg
(65.1% of theory) of the title compound.
1H NMR (200 MHz, DMSO-d 6): δ – 10.03 (s, IH, br), 9.17 (d, IH), 8.43 (s, IH), 7.98 (m, IH), 7.63 (m, IH ), 7.52 (dd, IH), 4.33 (m, IH), 3.77-3.10 (m, 6H), 2.28-
2.02 (m, 2H), 1.92 (m, 2H), 1.75 (m, IH) ppm.
HPLC: R t = 4.0 min (Method H)
MS (ESIpos): m / z = 321 (M + H) + (free base).
Example 70
N-[(3 S) – 1-azabicyclo [2.2.2] oct-3-yl]-7-chloro-1-benzothiophene-2-carboxamide hydrochloride UNDESIRED
x HCI
176.2 mg (0.83 mmol) of 7-chloro-l-benzothiophene-2-carboxylic acid, 150 mg (0.75 mmol) of S-3-Aminochinuklidin dihydrochloride, 343.7 mg (0.90 mmol) of HATU, 350.5 mg (2.71 mmol) of N, N- diisopropylethylamine and 3.0 ml of DMF are implemented according to the general procedure (Method B). The reaction mixture is purified by preparative HPLC. The product will be in a mixture of 4 M HCl solution in dioxane and methanol, and then concentrated. Obtained 231.9 mg (85.7% of theory) of the title compound. The analytical data are consistent with those of the enantiomeric compound from Example 69.
………………………………………
(Reference Example 3)
Synthesis of (R)-7-Chloro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide hydrochloride (hereinafter referred to as the compound of Reference Example 3):
Synthesis of (R)-7-Chloro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide hydrochloride (hereinafter referred to as the compound of Reference Example 3):
[First step]Synthesis of 7-Chloro-1-benzothiophene-2-carboxylic acid:
[Second step]Synthesis of (R)-7-Chloro-N-(quinuclidine-3-yl)benzo[b]thiophene-2-carboxamide:
To a solution (10 mL) of 7-chloro-1-benzothiophene-2-carboxylic acid (210 mg, 1.0 mmol) in chloroform, o-(benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate (570 mg, 1.5 mmol) and diisopropylethylamine (0.70 mL, 4.0 mmol) were added. Thereafter, (R)-quinuclidine-3-amine hydrochloride (200 mg, 1.0 mmol) was added thereto, and the resulting mixture was stirred at room temperature. Sixteen hours later, distilled water and 1.0 N aqueous sodium hydroxide solution were added thereto, and the resultant was extracted with chloroform. The organic layer was washed with brine, then dried over anhydrous sodium sulfate and concentrated. The obtained crude product was purified by silica gel column chromatography (amine silica gel DM1020, Fuji Silysia Chemical Ltd., chloroform alone to chloroform/methanol = 90/10) to obtain the title compound (170 mg; 53%) as a white solid.
1H-NMR (400 MHz, DMSO-d6)
δ: 1.22-1.38 (1H, m), 1.53-1.62 (2H, m), 1.75-1.82 (2H, m), 2.63-2.73 (4H, m), 2.84-2.94 (1H, m), 3.07-3.18 (1H, m), 3.90-4.00 (1H, m), 7.49 (1H, dd, J=7.6, 8.0 Hz), 7.59 (1H, d, J=7.6 Hz), 7.96 (1H, d, J=8.0 Hz), 8.31 (1H, s), 8.62-8.66 (1H, m).
MS (ESI) [M+H]+ 321
1H-NMR (400 MHz, DMSO-d6)
δ: 1.22-1.38 (1H, m), 1.53-1.62 (2H, m), 1.75-1.82 (2H, m), 2.63-2.73 (4H, m), 2.84-2.94 (1H, m), 3.07-3.18 (1H, m), 3.90-4.00 (1H, m), 7.49 (1H, dd, J=7.6, 8.0 Hz), 7.59 (1H, d, J=7.6 Hz), 7.96 (1H, d, J=8.0 Hz), 8.31 (1H, s), 8.62-8.66 (1H, m).
MS (ESI) [M+H]+ 321
[Third step]Synthesis of Compound of Reference Example 3:
To a solution (2.0 mL) of (R)-7-chloro-N-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide (170 mg, 0.53 mmol) in ethyl acetate, hydrogen chloride-ethyl acetate solution (4.0 M, 0.20 mL, 0.80 mmol) was added, and the resulting mixture was stirred at room temperature. Ten minutes later, the obtained solid was filtered off, washed with ethyl acetate and hexane, and dried to obtain the compound of Reference Example 3 (170 mg; 90%) as a white solid.
1H-NMR (400 MHz, DMSO-d6)
δ: 1.70-1.78 (1H, m), 1.86-1.94 (2H, m), 2.10-2.19 (2H, m), 3.18-3.35 (5H, m), 3.63-3.72 (1H, m), 4.27-4.36 (1H, m), 7.50 (1H, d, J=7.6, 8.0 Hz), 7.61 (1H, d, J=7.6 Hz), 7.98 (1H, d, J=8.0 Hz), 8.38 (1H, s), 9.07-9.10 (1H, m), 9.80-9.85 (1H, m).
MS (ESI) [M+H]+
321
1H-NMR (400 MHz, DMSO-d6)
δ: 1.70-1.78 (1H, m), 1.86-1.94 (2H, m), 2.10-2.19 (2H, m), 3.18-3.35 (5H, m), 3.63-3.72 (1H, m), 4.27-4.36 (1H, m), 7.50 (1H, d, J=7.6, 8.0 Hz), 7.61 (1H, d, J=7.6 Hz), 7.98 (1H, d, J=8.0 Hz), 8.38 (1H, s), 9.07-9.10 (1H, m), 9.80-9.85 (1H, m).
MS (ESI) [M+H]+
321
| WO1991012254A1* | 15 Feb 1991 | 17 Aug 1991 | Novo Nordisk As | Substituted urea compounds and their preparation and use |
| WO2004069141A2* | 5 Feb 2004 | 19 Aug 2004 | Strakan Ltd | Transdermal granisetron |
| WO2004076449A2* | 20 Feb 2004 | 10 Sep 2004 | Jozef Klucik | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| WO2008019372A2* | 7 Aug 2007 | 14 Feb 2008 | Amr Technology Inc | 2-aminobenzoxazole carboxamides as 5ht3 modulators |
| WO2008096870A1* | 8 Feb 2008 | 14 Aug 2008 | Astellas Pharma Inc | Aza-bridged-ring compound |
| JPH0881374A * | Title not available |
MW: 357.3032 | ||
| 2 |
MW: 320.8423 | |
| 3 |
MW: 375.318 |







If the products you look for are not in our catalog we would be pleased to offer our custom synthesis service. N-butyl-3-metylpyridinium hexafluorophosphate
ReplyDelete