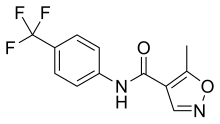
Leflunomide
RS-34821, SU-101, HWA-486, Arava,75706-12-6,
C12-H9-F3-N2-O2
270.2091
Antiarthritic Drugs, Brain Cancer Therapy, Disease-Modifying Drugs, IMMUNOMODULATING AGENTS, Immunosuppressants, Oncolytic Drugs, Ovarian Cancer Therapy, Prostate Cancer Therapy, Psoriatic Arthritis, Treatment of , Rheumatoid Arthritis, Treatment of, TREATMENT OF MUSCULOSKELETAL & CONNECTIVE TISSUE DISEASES, Treatment of Transplant Rejection, Dihydroorotate Dehydrogenase Inhibitors, Inhibitors of Signal Transduction Pathways, PDGFR Inhibitors
Launched-1998
Inhibits dihydroorotate dehydrogenase, the fourth enzyme in the pyrimidine biosynthetic pathway; antagonizes growth-factor mediated smooth muscle cell proliferation in vitro.
- Arava
- HSDB 7289
- HWA 486
- HWA-486
- Leflunomida
- Leflunomida [INN-Spanish]
- Leflunomide
- Leflunomidum
- Leflunomidum [INN-Latin]
- SU 101 (pharmaceutical)
- SU101
- UNII-G162GK9U4W
Leflunomide (brand names: Arabloc, Arava, Lunava, Repso) is an immunosuppressive disease-modifying antirheumatic drug (DMARD),[2] used in active moderate to severe rheumatoid arthritis and psoriatic arthritis. It is apyrimidine synthesis inhibitor.[3]
Medical use
Rheumatoid arthritis and psoriatic arthritis are the only indications that have received regulatory approval.[1][4] Clinical studies regarding the following diseases have been conducted:[5]
- Polyoma BK Virus Nephropathy[6]
- Kimura’s disease[7]
- Systemic lupus erythematosus[8]
- Felty’s syndrome [9]
- Takayasu arteritis[10]
- Wegener’s granulomatosis[9]
- Ankylosing spondylitis[11]
- Crohn’s disease[12][13]
- Sarcoidosis[14]
- Uveitis[15]
- Still’s disease[16]
- Prostate cancer[17]
- Pemphigoid[18]
- Prevention of organ transplant rejection[19]
Side effects
Its principle dose-limiting side effects are liver damage, lung disease and immunosuppression.[19] The most common side effects (occurring in >1% of those treated with it) are, in approximately descending order of frequency:[1][4][20][21][22][23][24] diarrhoea, respiratory tract infections, hair loss, high blood pressure, rash, nausea, bronchitis, headache, abdominal pain, abnormal liver function tests, back pain, indigestion, urinary tract infection, dizziness, infection, joint disorder, itchiness, weight loss, loss of appetite, cough, gastroenteritis,pharyngitis, stomatitis, tenosynovitis, vomiting, weakness, allergic reaction, chest pain, dry skin, eczema,paraesthesia, pneumonia, rhinitis, synovitis,cholelithiasis and shortness of breath. Whereas uncommon side effects (occurring in 0.1-1% of those treated with the drug) include:[4] constipation, oralthrush, stomatitis, taste disturbance, thrombocytopenia and hives. Rarely (in 0.1% of those treated with it) it can cause:[4] anaphylaxis, angiooedema,anaemia, agranulocytosis, eosinophilia,leucopenia, pancytopenia, vasculitis,toxic epidermal necrolysis, Stevens-Johnson syndrome, cutaneous lupus erythematosus, severe infection, interstitial lung disease, cirrhosis and liver failure.
Contraindications
Contraindications include:[1]
- Pregnancy, women of childbearing potential (unless contraception used)
- Liver disease, hepatitis B/Cseropositive
- Active serious infections
- Hypersensitivity
Interactions
Other immunomodulatory treatments should be avoided due to the potential for additive immunosuppressant effects, or in the case of immunostimulants likeechinacea or astragalus, reduced therapeutic effects.[1] Likewise live vaccines (like haemophilus influenzae type b vaccine and yellow fever vaccines) should be avoided due to the potential for severe infection due to the immunosuppressive nature of the treatment.[1]
The concomitant use of methotrexate, in particular, may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy leflunomide plus methotrexate.[25]However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.[25]
Mechanism of action
Leflunomide is an immunomodulatory drug that achieves its effects by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase(an enzyme involved in de novo pyrimidine synthesis) (abbreviation DHODH), which plays a key role in the de novo (from scratch) synthesis of the uridine monophosphate (rUMP), which is required for the synthesis of DNA and RNA, hence leflunomide inhibits the reproduction of rapidly dividing cells, especially lymphocytes.[19] The inhibition of human DHODH by teriflunomide, the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of rheumatoid arthritis (RA).[26] Teriflunomide also inhibits severaltyrosine kinases.[19] Teriflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on de novo synthesis.[26] Teriflunomide also has antiviral effects against numerous viruses including CMV, HSV1 and the BK virus, which it achieves by inhibiting viral replication by interfering with nucleocapsidtegumentation and hence virion assembly.[19]
Pharmacokinetics
It has an oral bioavailability of 80%, protein binding of >99%, metabolism sites of the GI mucosa and liver, volume of distribution (Vd) of 0.13 L/kg, elimination half-life of 14-18 days and excretion routes of faeces (48%) and urine (43%).[19][1][20]
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| 5-methyl-N-[4-(trifluoromethyl) phenyl]-isoxazole-4-carboxamide | |
| CLINICAL DATA | |
| TRADE NAMES | Arabloc, Arava, Lunava, Repso |
| AHFS/DRUGS.COM | monograph |
| MEDLINEPLUS | a600032 |
| LICENCE DATA | EMA:Link, US FDA:link |
| PREGNANCY CAT. | |
| LEGAL STATUS | |
| ROUTES | Oral (tablets) |
| PHARMACOKINETIC DATA | |
| BIOAVAILABILITY | 80%[1] |
| PROTEIN BINDING | >99%[1] |
| METABOLISM | GI mucosa and liver[1] |
| HALF-LIFE | 14-18 days[1] |
| EXCRETION | Faeces (48%), urine (43%)[1] |
| IDENTIFIERS | |
| CAS NUMBER | 75706-12-6 |
| ATC CODE | L04AA13 |
| PUBCHEM | CID 3899 |
| DRUGBANK | DB01097 |
| CHEMSPIDER | 3762 |
| UNII | G162GK9U4W |
| KEGG | D00749 |
| CHEBI | CHEBI:6402 |
| CHEMBL | CHEMBL960 |
| CHEMICAL DATA | |
| FORMULA | C12H9F3N2O2 |
| MOL. MASS | 270.207 g/mol |
……………………………
5-Substd. 4-isoxazolecarboxamides with platelet antiaggregating and other activities
Leflunomide can be obtained by several related ways: 1) The reaction of diketene (I) with 4-(trifluoromethyl)-aniline (II) in hot acetonitrile gives N-[4-(trifluoro-methyl) phenyl]acetoacetamide (III) , which by reaction with triethyl orthoformate (IV) in refluxing acetic anhydride yields the corresponding ethoxymethylene derivative (V). Finally, this compound is cyclized with hydroxylamine in refluxing ethanol/water. 2) The reaction of ethyl acetoacetate (VI) with triethyl orthoformate (IV) as before gives the corresponding ethoxymethylene derivative (VII), which by cyclization with hydroxylamine as before affords 5-methylisoxazole-4-carboxylic acid ethyl ester (VIII). The hydrolysis of (VIII) under acidic conditions yields the free acid (IX), which is converted into the acid chloride (X) by standard methods. Finally, this compound is condensed with 4-(trifluoro-methyl)aniline (II) by means of triethylamine in acetonitrile. 3) The formation of leflunomide from acid (IX) or its derivatives such as ethyl (VIII) or other esters can also be performed through other standard procedures of amide formation. 4) The N-[4-(trifluoromethyl)phenyl]acetoacetamide (III) can also be obtained by reaction of 4-(trifluoro-methyl) aniline (II) with 2,2,6-trimethyl-4H-1,3-dioxin-4-one (XI) in refluxing xylene.

…………………………..
Leflunomide is a pyrimidine synthase inhibitor of the DMARD-type (disease-modifying anti-rheumatic drug) marketed by Sanofi-Aventis. Unlike NSAIDs, which only deal with symptoms of rheumatoid arthritis, DMARDs target the cause of it. DMARDs are not necessarily structurally or mechanistically related. The effect of leflunomide is possibly due to its regulation of the immune system via affecting lymphocytes. Its synthesis [134] is relatively straightforward starting with a Knoevenagel condensation of ethyl acetoacetate (39) and triethyl orthoformate in the presence of acetic anhydride. The resulting ethyl ethoxymethylene acetoacetate (448) is next condensed with hydroxylamine hydrate in methanol to yield ethyl 5-methylisoxazole-4-carboxylate (449). The ethyl ester is hydrolysed under acidic conditions and the carboxylic acid activated with thionyl chloride in DMF for amide formation with 4-trifluoromethylaniline (450) (Scheme 86).
Scheme 86: Synthesis of leflunomide.
Ramakrishnam, A.; Gobind, K.; Neeraj, K.; Dnyaneshwar, S. An Improved Process for Preparation of Leflunomides. WO Patent 2007/086076, Aug 2, 2007.
…………………………………
US patent 5,494,911 discloses process for preparation of Teriflunomide in Example- 4 as shown in given below scheme-I.
References
- “Arava (leflunomide) dosing, indications, interactions, adverse effects, and more”. Medscape Reference. WebMD. Retrieved 11 March 2014.
- Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P (January 2005).“When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide?”. Annals of the rheumatic diseases 64 (1): 44–51.doi:10.1136/ard.2003.016709.PMC 1755199. PMID 15271770.
- Pinto P, Dougados M (2006). “Leflunomide in clinical practice”. Acta reumatológica portuguesa 31 (3): 215–24. PMID 17094333.
- ^ Jump up to:a b c d Rossi, S, ed. (2013). Australian Medicines Handbook(2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust.ISBN 978-0-9805790-9-3.
- Jump up^ http://clinicaltrials.gov/ct2/results?term=Leflunomide
- Jump up^ Blanckaert, K; De Vriese, AS (23 September 2006). “Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients” (PDF). Nephrology Dialysis Transplantation 21 (12): 3364–3367.doi:10.1093/ndt/gfl404.
- Jump up^ Dai, L; Wei, XN; Zheng, DH; Mo, YQ; Pessler, F; Zhang, BY (June 2011). “Effective treatment of Kimura’s disease with leflunomide in combination with glucocorticoids.”.Clinical Rheumatology 30 (6): 859–65.doi:10.1007/s10067-011-1689-2.PMID 21286771.
- Jump up^ Wu, GC; Xu, ; Huang, Q; Wu, H (February 2013). “Leflunomide: friend or foe for systemic lupus erythematosus?”.Rheumatology International 33 (2): 273–6.doi:10.1007/s00296-012-2508-z. PMID 22961090.
- ^ Jump up to:a b Sanders, S; Harisdangkul, V (2002). “Leflunomide for the treatment of rheumatoid arthritis and autoimmunity”. American Journal of Medical Sciences 323 (4): 190–3.doi:10.1097/00000441-200204000-00004. PMID 12003373.
- Jump up^ Unizony, S; Stone, JH; Stone, JR (January 2013). “New treatment strategies in large-vessel vasculitis.”. Current Opinion in Rheumatology 25 (1): 3–9.doi:10.1097/BOR.0b013e32835b133a.PMID 23114585.
- Jump up^ Haibel, H; Rudwaleit, M; Braun, J; Sieper, J (January 2005).“Six months open label trial of leflunomide in active ankylosing spondylitis.” (PDF). Annals of the Rheumatic Diseases 64 (1): 124–6.doi:10.1136/ard.2003.019174. PMC 1755172.PMID 15608310.
- Jump up^ Prajapati, DN; Knox, JF; Emmons, J; Saeian, K; Csuka, ME; Binion, DG (August 2003). “Leflunomide treatment of Crohn’s disease patients intolerant to standard immunomodulator therapy.”. Journal of Clinical Gastroenterology 37 (2): 125–8.doi:10.1097/00004836-200308000-00006. PMID 12869881.
- Jump up^ Holtmann, MH; Gerts, AL; Weinman, A; Galle, PR; Neurath, MF (April 2008). “Treatment of Crohn’s disease with leflunomide as second-line immunosuppression : a phase 1 open-label trial on efficacy, tolerability and safety.”. Digestive Diseases and Sciences 53 (4): 1025–32. doi:10.1007/s10620-007-9953-7. PMID 17934840.
- Jump up^ Panselinas, E; Judson, MA (October 2012). “Acute pulmonary exacerbations of sarcoidosis.” (PDF). Chest 142 (4): 827–36.doi:10.1378/chest.12-1060.PMID 23032450.
- Jump up^ Roy, M (August 2007). “Early clinical experience with leflunomide in uveitis.”. Canadian Journal of Ophthalmology 42 (4): 634.doi:10.3129/canjophthalmol.i07-085.PMID 17641721.
- Jump up^ Pirildar, T (May 2003). “Treatment of adult-onset Still’s disease with leflunomide and chloroquine combination in two patients.”.Clinical Rheumatology 22 (2): 157.doi:10.1007/s10067-002-0667-0.PMID 12740686.
- Jump up^ “Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer”. ClinicalTrials.gov. National Institute of Health. September 2012. Retrieved 11 March 2014.
- Jump up^ “Leflunomide Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL)”. ClinicalTrials.gov. National Institute of Health. December 2008. Retrieved 11 March 2014.
- ^ Jump up to:a b c d e f Teschner, S; Burst, V (September 2010). “Leflunomide: a drug with a potential beyond rheumatology.”.Immunotherapy 2 (5): 637–50. doi:10.2217/imt.10.52.PMID 20874647.
- ^ Jump up to:a b “PRODUCT INFORMATION ARAVA®” (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. 7 August 2012. Retrieved 11 March 2014.
- Jump up^ “Arava : EPAR – Product Information” (PDF). European Medicines Agency. Sanofi-Aventis Deutschland GmbH. 21 November 2013. Retrieved 11 March 2014.
- Jump up^ “Data Sheet Arava®” (PDF). Medsafe. sanofi-aventis new zealand limited. 29 June 2012. Retrieved 11 March 2014.
- Jump up^ “ARAVA (leflunomide) tablet, film coated [sanofi-aventis U.S. LLC]“. DailyMed. sanofi-aventis U.S. LLC. November 2012. Retrieved 11 March 2014.
- Jump up^ “Arava 100mg Tablets – Summary of Product Characteristic”.electronic Medicines Compendium. SANOFI. 21 February 2014. Retrieved 11 March 2014.
- ^ Jump up to:a b Lee, S.; Park, Y.; Park, J.; Kang, Y.; Nam, E.; Kim, S.; Lee, J.; Yoo, W.; Lee, S. (2009). “Combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis”.Scandinavian journal of rheumatology 38 (1): 11–14.doi:10.1080/03009740802360632. PMID 19191187.
- ^ Jump up to:a b Fox, RI; Herrmann, ML; Frangou, CG; Wahl, GM; Morris, RE; Strand, V; Kirschbaum, BJ (December 1999). “Mechanism of action for leflunomide in rheumatoid arthritis.”. Clinical Immunology 93 (3): 198–208. doi:10.1006/clim.1999.4777.PMID 10600330.
External links
- National Rheumatoid Arthritis Society (NRAS) Information about Disease Modifying drugs such as Leflunomide
- http://www.arava.com/professional/home.do (full prescribing information)
- http://www.rheuma-online.de/medikamente/leflunomid-arava/studien-zu-leflunomid-arava/gibt-es-untersuchungen-zu-leflunomid-in-weiteren-einsatzgebieten.html (in German, regarding potential indications)
- http://www.arznei-telegramm.de/register/0204507.pdf (in German, regarding discontinuation of the drug)
- http://www.emea.europa.eu/pdfs/human/press/pus/561101en.pdf(warning as of 2001 regarding hepatotoxicity) (URL DEAD 16 Oct 2010)
- The safety of leflunomide Australian Prescriber
Dosages/Routes/Forms
by SANOFI AVENTIS US
| STRENGTH | FORM/ROUTE | MARKETING STATUS | RLD | TE CODE |
|---|---|---|---|---|
| 10MG | TABLET;ORAL | 1 | 0 | AB |
| 20MG | TABLET;ORAL | 1 | 1 | AB |
| 100MG | TABLET;ORAL | 1 | 1 |
Approval History
2000-09-21
005
Control Supplement
2000-02-23
004
Labeling Revision
1999-07-20
002
Package Change Package Change
1998-12-11
001
Manufacturing Change or Addition
1998-09-10
000
Approval

Spectra
UV – spectrum
IR – spectrum
Links
- UV and IR Spectra. H.-W. Dibbern, RM Muller, E. Wirbitzki, 2002 ECV
- NIST / EPA / NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman.Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
LEFLUNOMIDE IMPURITY C [EP
5-Methyl-N-(3-(trifluoromethyl)phenyl)isoxazole-4-carboxamide
LEFLUNOMIDE IMPURITY D [EP]
5-Methylisoxazole-4-carboxylic acid
LEFLUNOMIDE IMPURITY E [EP]
3-Methyl-N-(4-(trifluoromethyl)phenyl)isoxazole-4-carboxamide
LEFLUNOMIDE IMPURITY F [EP]
5-Methyl-N-(2-(trifluoromethyl)phenyl)isoxazole-4-carboxamide
LEFLUNOMIDE IMPURITY G [EP]
5-Methyl-N-(4-methylphenyl)isoxazole-4-carboxamide
LEFLUNOMIDE IMPURITY H [EP]
2-Cyano-N-(4-(trifluoromethyl)phenyl)acetamide


![[1860-5397-7-57-i86]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i86.png?max-width=550&background=EEEEEE)









We make the custom synthesis process more efficient and cost effective while maintaining the highest standards of quality and reliability. 1-decyl-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)imide
ReplyDelete