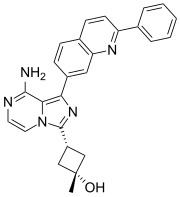
MAROPITANT
(7R,8S)-N-[(5-tert-Butyl-2-methoxyphenyl)methyl]-7-[di(phenyl)methyl]-1-azabicyclo[2.2.2]octan-8-amine
(2S,3S)-N-[(5-tert-butyl-2-methoxy-phenyl)methyl]-2-(diphenylmethyl)-1-azabicyclo[2.2.2]octan-3-amine
147116-67-4
PRECLINICAL, PFIZER
Maropitant, is described in WO1992021677, US 6,222,038 and US
6,255,230,US 5340826, US 5393762, EP 0769300, WO 2000073304, WO 2005082419, WO 2005082366
.................................................................................................
MAROPITANT CITRATE MONOHYDRATE
359875-09-5,
- Cerenia
- CJ-11,972
- Maropitant citrate
- UNII-LXN6S3999X
Maropitant (trade name Cerenia in the US and other countries), used as maropitantcitrate (USAN), is a neurokinin (NK1) receptor antagonist, which was developed by Zoetisspecifically for the treatment of motion sickness and vomiting in dogs. It was approved by the FDA in 2007 for use in dogs,[1][2] and more recently has also been approved for use in cats.[3]
MORE............
Use of the cryopreserved human hepatocyte sandwich-culture model to measure hepatic metabolism and biliary efflux
1st Int Conf Drug Des Disc (February 4-7, Dubai) 2008, Abst P-140
1st Int Conf Drug Des Disc (February 4-7, Dubai) 2008, Abst P-140
Proposed international nonproprietary names (Prop. INN): List 90
WHO Drug Inf 2004, 18(1): 56
WHO Drug Inf 2004, 18(1): 56
Maropitant, a NK-1 antagonist decreases the sevoflurane MAC during visceral stimulation in dogs
13th World Congr Pain (August 29-September 2, Montreal) 2010, Abst PW 320
13th World Congr Pain (August 29-September 2, Montreal) 2010, Abst PW 320
Identification of metabolites from maropitant using a dual-pressure linear ion trap and mass frontier software
9th Int ISSX Meet (September 4-8, Istanbul) 2010, Abst P343
9th Int ISSX Meet (September 4-8, Istanbul) 2010, Abst P343
Effect of maropitant, a new NK-1 receptor antagonist, on the sevoflurane minimum alveolar concentration during ovarian stimulation in cats
Annu Meet Am Soc Anesthesiol (ASA) (October 15-19, Chicago) 2011, Abst A1585
Annu Meet Am Soc Anesthesiol (ASA) (October 15-19, Chicago) 2011, Abst A1585
| US8183230 | 5-23-2012 | Antimicrobial preservatives to achieve multi-dose formulation using beta-cyclodextrins for liquid dosage forms |
| US2009099364 | 4-17-2009 | Process for preparation of 1-(2s,3s)-2-benzhydryl-n-(5- tert-butyl-2-methoxybenzyl)quinuclidin-3-amine |
| US2007155782 | 7-6-2007 | Nk-1 receptor antagonists anesthesia recovery |
| US2007129328 | 6-8-2007 | Pharmaceutical compositions of neurokinin receptor antagonists and cyclodextrin and methods for improved injection site toleration |
| US2003139443 | 7-25-2003 | Use of tachykinin antagonists, including NK-1 receptor antagonists, to modify unwanted behavior in dogs, cats and horses |
| US6255320 | 7-4-2001 | Polymorphs of a crystalline azo-bicyclo (2,2,2) octan-3-amine citrate and their pharmaceutical compositions |
| US5990125 | 11-24-1999 | NK-1 receptor antagonists for the treatment of cancer |
| EP0790825 | 8-28-1997 | NK-1 RECEPTOR ANTAGONISTS FOR THE TREATMENT OF EYE DISORDERS |
| WO9713514 | 4-18-1997 | NK-1 RECEPTOR ANTAGONISTS FOR PREVENTION OF NEUROGENIC INFLAMMATION IN GENE THERAPY |
| US5576317 | 11-20-1996 | NK-1 receptor antagonists and 5HT3 receptor antagonists for the treatment of |
| WO9614845 | 5-24-1996 | NK-1 RECEPTOR ANTAGONISTS FOR THE TREATMENT OF EYE DISORDERS |
| US5519033 | 5-22-1996 | Azabicyclo derivatives for treatment of urinary incontinence |
| US5393762 | 2-29-1995 | Pharmaceutical agents for treatment of emesis |
| US5340826 | 8-24-1994 | Pharmaceutical agents for treatment of urinary incontinence |
| WO9221677 | 12-11-1992 | bibNUCLIDINE DERIVATIVES |
anhydrous (2S,3S)-N-(methoxy-5-t-butylphenylmethyl-2-diphenylmethyl-1-azobicyclo[2,2,2] octan-3-amine citrate monohydrate salt, its single crystalline polymorphic Form A, and pharmaceutical composition containing them. The invention is also directed to a CNS active NK-1 receptor antagonist for treating emesis in a mammal including humans. Treating is defined here as preventing and treating.
U.S. Pat. No. 5,393,762 and U.S. Ser. No. 08/816,016, both incorporated by reference, describe pharmaceutical compositions and treatment of emesis using NK-1 receptor antagonists. The citrate monohydrate has significantly enhanced stability over other salt forms such as the benzoate which was unstable even at 5° C. The mesylate form is deliquescent.
U.S. Pat. No. 5,393,762 and U.S. Ser. No. 08/816,016, both incorporated by reference, describe pharmaceutical compositions and treatment of emesis using NK-1 receptor antagonists. The citrate monohydrate has significantly enhanced stability over other salt forms such as the benzoate which was unstable even at 5° C. The mesylate form is deliquescent.
synthesis
U.S. 5,807,867, U.S. 6,222,038 and U.S. 6,255,320.

The compound of Formula I, an NK1 receptor antagonist, is effective as an anti-emetic agent for mammals. The compound of Formula I is the subject of U.S. Pat. No. 6,222,038 and U.S. Pat. No. 6,255,320, and the preparation of the compound of Formula I is described therein. U.S. Pat. No. 5,393,762 also describes pharmaceutical compositions and treatment of emesis using NK-1 receptor antagonists. The multiple-use formulation of the compound of Formula I may be parenterally administrated for about five days at the same site for treatment of emesis or other indications. Intravenous or, preferably, subcutaneous administration is desirable for acute use, since retention and absorption of an oral dosage form may be problematic during bouts of emesis. The multiple-use formulation is described in a co-pending U.S. provisional application No. 60/540,897 assigned to and owned by Pfizer. Inc.
The compound of Formula I also improves anesthesia recovery in mammals. A co-pending U.S. provisional application No. 60/540,697 assigned to and owned by Pfizer Inc., describes a method of improving anesthesia recovery by administering a NK-1 antagonist prior to, during or after the administration of general anesthesia.
...........................................
US20090099364



Preparation of (2S,3S)-2-benzhydryl-N-(5-tert-butyl-2-methoxybenzyl) quinuclidin-3-amine citrate monohydrate, Compound of Formula Ia Step C, Scheme II
A solution of (2S,3S)-2-benzhydryl-N-(tert-butyl-2-methoxybenzyl) quinuclidin-3-amine (33.95 kg, 72.4 moles) and anhydrous citric acid (15.3 kg, 79.7 moles) in a mixture of acetone (215 kg) and water (13.6 kg) was heated to 38-42° C. The resultant mixture was then transferred to another reactor via an in-line filter. The transfer line and filter were washed through with acetone (54 kg) and these filtered washings were added to the solution. The resultant mixture was then cooled to 20-25° C. and filtered fart-butyl methyl ether (252 kg) was added portion-wise over a period of approximately 35 minutes. The resultant suspension was then granulated at 20-25° C. for approximately 20 hours. The solid was then collected by filtration on an agitated filter-dryer and the filter cake was washed twice with filtered tert-butyl methyl ether (50 kg each). The resultant solid was then dried at 35° C. under vacuum with agitation to give the title compound (44.4 kg) as a colourless solid. The product was then milted.
1H-NMR (500 MHz, d6-methanol, 30° C.) δ: 7.46 (2H, d), 7.45 (2H, d), 7.37 (4H, m), 7.31 (1H, m), 7.29 (1H, m), 7.24 (1H, dd), 6.95 (1H, d), 6.76 (1H, d), 4.75 (1H, dd), 4.71 (1H, d), 3.76 (1H, m), 3.57 (1H, d), 3.55 (3H, s), 3.37 (1H, m), 3.31 (1H, m), 3.26 (1H, m), 3.24 (1H, d), 3.10 (1H, t), 2.83 (2H, d), 2.75 (2H, d), 2.51 (1H, m), 2.35 (1H, m), 2.11 (1H, m), 2.06 (1H, m), 1.85 (1H, m), 1.29 (9H, s).
13C NMR (125.7 MHz, d6-methanol, 30° C.) δ: 179.4, 175.0, 156.8, 144.0, 141.5, 141.4, 131.1, 130.6, 129.4, 128.9, 128.7, 128.3, 128.2, 127.2, 126.4, 111.0, 74.0, 64.7, 56.1, 54.2, 50.4, 48.5, 48.3, 44.9, 43.8, 34.8, 32.9, 25.3, 22.2, 18.1.
LRMS (ES+): m/z [MH+] 469.


The compound of Formula I also improves anesthesia recovery in mammals. A co-pending U.S. provisional application No. 60/540,697 assigned to and owned by Pfizer Inc., describes a method of improving anesthesia recovery by administering a NK-1 antagonist prior to, during or after the administration of general anesthesia.
...........................................
US20090099364



Preparation of (2S,3S)-2-benzhydryl-N-(5-tert-butyl-2-methoxybenzyl) quinuclidin-3-amine citrate monohydrate, Compound of Formula Ia Step C, Scheme II
A solution of (2S,3S)-2-benzhydryl-N-(tert-butyl-2-methoxybenzyl) quinuclidin-3-amine (33.95 kg, 72.4 moles) and anhydrous citric acid (15.3 kg, 79.7 moles) in a mixture of acetone (215 kg) and water (13.6 kg) was heated to 38-42° C. The resultant mixture was then transferred to another reactor via an in-line filter. The transfer line and filter were washed through with acetone (54 kg) and these filtered washings were added to the solution. The resultant mixture was then cooled to 20-25° C. and filtered fart-butyl methyl ether (252 kg) was added portion-wise over a period of approximately 35 minutes. The resultant suspension was then granulated at 20-25° C. for approximately 20 hours. The solid was then collected by filtration on an agitated filter-dryer and the filter cake was washed twice with filtered tert-butyl methyl ether (50 kg each). The resultant solid was then dried at 35° C. under vacuum with agitation to give the title compound (44.4 kg) as a colourless solid. The product was then milted.
1H-NMR (500 MHz, d6-methanol, 30° C.) δ: 7.46 (2H, d), 7.45 (2H, d), 7.37 (4H, m), 7.31 (1H, m), 7.29 (1H, m), 7.24 (1H, dd), 6.95 (1H, d), 6.76 (1H, d), 4.75 (1H, dd), 4.71 (1H, d), 3.76 (1H, m), 3.57 (1H, d), 3.55 (3H, s), 3.37 (1H, m), 3.31 (1H, m), 3.26 (1H, m), 3.24 (1H, d), 3.10 (1H, t), 2.83 (2H, d), 2.75 (2H, d), 2.51 (1H, m), 2.35 (1H, m), 2.11 (1H, m), 2.06 (1H, m), 1.85 (1H, m), 1.29 (9H, s).
13C NMR (125.7 MHz, d6-methanol, 30° C.) δ: 179.4, 175.0, 156.8, 144.0, 141.5, 141.4, 131.1, 130.6, 129.4, 128.9, 128.7, 128.3, 128.2, 127.2, 126.4, 111.0, 74.0, 64.7, 56.1, 54.2, 50.4, 48.5, 48.3, 44.9, 43.8, 34.8, 32.9, 25.3, 22.2, 18.1.
LRMS (ES+): m/z [MH+] 469.


THANKS AND REGARD'S
DR ANTHONY MELVIN CRASTO Ph.D
DR ANTHONY MELVIN CRASTO Ph.D
GLENMARK SCIENTIST , NAVIMUMBAI, INDIA
did you feel happy, a head to toe paralysed man's soul in action for you round the clock
need help, email or call me
MOBILE-+91 9323115463
web link
I was paralysed in dec2007, Posts dedicated to my family, my organisation Glenmark, Your readership keeps me going and brings smiles to my family