Whisky Lactone
Whisky lactone, also known as β-methyl-γ-octalactone or quercus lactone (from the Latin for oak treeQuercus alba), is a flavouring found in American bourbon whiskies, and is also found in all types of oak. The flavour gets into the whisky when it’s matured in oak barrels. The pure molecule has a fierce, strong, and sweet smell and can be dissolved in alcohol in any proportion.
6
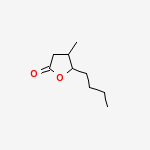
5-butyl-4-methyloxolan-2-one | CAS Registry Number: 39212-23-2
Synonyms: Whiskey lactone, Oaklactone, 3-Methyl-4-octanolide, cis-3-Methyl-4-octanolide, beta-Methyl-gamma-octalactone, W380318_ALDRICH, FEMA No. 3803, EINECS 254-357-4, 5-Butyl-4-methyldihydro-2(3H)-furanone, 5-Butyldihydro-4-methyl-2(3H)-furanone, cis-.beta.-Methyl-.gamma.-Octalactone, 2(3H)-Furanone, 5-butyldihydro-4-methyl-, 4-Hydroxy-3-methyloctanoic acid lactone, 5-Butyldihydro-4-methylfuran-2(3H)-one, 5-Butyl-4-methyl-dihydro-2(3H)-furanone, ()-5-Butyl-4-methyldihydro-2(3H)-furanone, 2(3H)-Furanone, 5-butyldihydro-4-methyl-, cis-, 39212-23-2
The cis isomer is the chemical extracted from oak wood that gives whiskey a coconut-like aroma. But not all isomers of this molecule are quite this tasty. The trans isomers of 3-methyl-4-octanolide is by contrast, used as an insect repellent.

Whiskey lactone (3-methyl-4-octanolide) is one of perfume components of whiskey and wine.
There are stereoisomers in the natural whiskey lactone, which are of the trans-type or cis-type in accordance with the configuration of methyl group at 3-position thereof and butyl group at 4-position thereof.
As compared to trans-whiskey lactone
[(3S,4R)-3-methyl-4-octanolide] (D), generally, a less amount of cis-whiskey lactone
[(3S,4S)-3-methyl-4-octanolide] (A) is contained, for example, in whiskey and wine. However, cis-whiskey lactone (A) is superior in the characteristic of perfume.
However, means for selectively synthesizing natural type cis-whiskey lactone (A) having superior properties than those of trans-type isomer (D) as described above has not been known.
Liebigs Annalen der Chemie, No. 12, 1986, pages 2112 – 2122, disclose a method, how all four stereoisomers of 3-methyl-4-octanolide can be produced. It is taught to separate a racemic cis/trans octanolide into the two cis- and the two trans-isomers chromatographically. Furthermore, it is disclosed how to hydrolyse a lactone ring with potassium hydroxide and to protect the carboxy group subsequently yielding the isopropyl carboxylic ester. Diastereomeric pairs of the acid-protected gamma-hydroxyacid are formed by reaction with an optically active carboxylic acid, and are separated from each other by liquid chromatography. It is disclosed how to perform the hydrolysis of the diastereomers and the relactonization to obain the four stereoisomers of whiskey-lactone. No reaction at the 4-hydroxy group is suggested to invert the configuration of the chiral centre
Synthesis, No. 1, 1981, pages 1-28, discloses the use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. However, there is no indication for a combination of these documents. Furthermore, it is well-known that the Mitsunobu reaction proceeds according to Sn2 reaction mechanism. In the case of 3,4-trans compound (III) which is a substrate of the method of the present invention, the steric hindrance to the 4-methyl group is large because of the influence from the methyl group at a 3-position.
Physical data of the obtained cis-whiskey lactone are
Boiling Point : 124 – 126°C/(17 mmHg) 2266 Pa
¹H-NMR(CDCl₃) : δ 0.92(3H, t, J = 7.0 Hz), 1.02(3H, d, J = 6.9 Hz), 1.20 – 1.75(6H, m), 2.20(1H, dd, J = 3.8 and 16.8 Hz), 2.51 – 2.64(1H, m), 2.70(1H, dd, J = 7.8 and 16.8 Hz), 4.40 – 4.48(1H, m) (ppm units)
|



No comments:
Post a Comment