Oct252013

http://www.bayer.com/
Bayer
With 11 treatments in Phase I trials, 8 in Phase II, and 13 in Phase III, Bayer has a strong pipeline.
By far the most interest currently, given that the latest reports came out October 21st, is riociguat (BAY 63-2521),
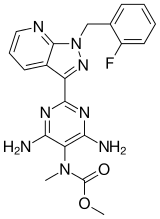
which has had good news from its ongoing Phase III clinical trials of the treatment for pulmonary arterial hypertension, also known as PAH. PAH is a progressive condition that overburdens the heart.
Trials indicate subjects had improved heart function and could better tolerate physical exercise. Patients on riociguat improved their walking distance by 36 meters on average, while those on placebo showed no improvement.
Professor Hossein Ardeschir Ghofrani of University Hospital Giessen, the principal investigator, was quite pleased with the results and explained the value of the measurement. “The six-minute walk distance test is a well-validated clinical measure in patients with PAH, and therefore, the results of the PATENT-1 trial are encouraging. . .These data from the PATENT study suggest that riociguat may be a potential treatment option both for patients who have never been treated for PAH as well as for those who have received prior treatment.”
Hossein A. Ghofrani
Associate Professor of Internal Medicine,
MD (University of Giessen) 1995 Research interests: pulmonary hypertension, ischaemia-reperfusion, experimental therapeutics, clinical trials
Associate Professor of Internal Medicine,
MD (University of Giessen) 1995 Research interests: pulmonary hypertension, ischaemia-reperfusion, experimental therapeutics, clinical trials
Although Bayer put forth no sales estimate for the treatment, analysts predicted 2017 sales from riociguat of $480 million.
BAYER PIPELINE AS ON OCT 25 2013
phase 1
| Project | Indication |
|---|---|
| CDK-Inhibitor (BAY 1000394) | Cancer |
| Mesothelin-ADC (BAY 94-9343) | Cancer |
| PSMA Bi TE Antibody (BAY 2010112) | Cancer |
| PI3K-Inhibitor (BAY 1082439) | Cancer |
| FGFR2 Antibody (BAY 1179470) | Cancer |
| HIF-PH (BAY 85-3934) | Anemia |
| Partial Adenosine A1 Agonist(BAY 1067197) | Heart Failure |
| Vasopressin Receptor Antagonist(BAY 86-8050) | Heart Failure |
| sGC Stimulator (BAY 1021189) | Heart Failure |
| S-PRAnt (BAY 1002670) | Symptomatic uterine fibroids |
| BAY 1026153 | Endometriosis |
phase2
| Project | Indication |
|---|---|
| PI3K-Inhibitor (BAY 80-6946) | Cancer |
| Regorafenib | Cancer |
| Refametinib (MEK-Inhibitor) | Cancer |
| Radium-223-Dichloride | Cancer |
| Sorafenib | Additional Indications |
| MR-Antagonist (BAY 94-8862) | Congestive Heart Failure (CHF) |
| MR-Antagonist (BAY 94-8862) | Diabetic Nephopathy |
| Riociguat (sGC Stimulator) | Pulmonary Hypertension |
| Neutrophil Elastase Inhibitor(BAY 85-8501) | Bronchiectasis |
phase 3
| Project | Indication |
|---|---|
| Sorafenib | Breast Cancer |
| Sorafenib | Adjuvant HCC |
| Sorafenib | Adjuvant RCC |
| Regorafenib | HCC 2nd line |
| Rivaroxaban | Major Adverse Cardiac Events |
| Rivaroxaban | CHF and CAD |
| peg rFVIII(BAY 94-9027) | Hemophilia |
| Aflibercept | Myopic choroidal neovascularization (mCNV) |
| Aflibercept | Diabetic Macular Edema (DME) |
| LCS 16 | Contraception |
| Vaginorm | Vulvovaginal atrophy (VVA) |
| Sodium Deoxycholate | Submental fat removal |
| Cipro DPI | Lung infection |
| Tedizolid | Skin and Lung Infections |
| Amikacin Inhale | Gram-negative pneumonia |
Information for Download from bayer
Sorafenib tosylate
TEDIZOLID PHOSPHATE
Bayer Accelerates Clinical Development of Promising New Drug Candidates
Five new molecular entities projected to enter Phase III by 2015 / Addressing unmet medical needs in the areas of oncology, cardiology, and women’s health / Initiation of further studies with recently launched products planned to add new treatment options
Leverkusen, October 8, 2013 – Following the recent commercial introduction of five new drugs to address the medical needs of patients with various diseases, Bayer is now accelerating the development of further five promising drug candidates which are currently undergoing phase I and II clinical studies. The company today announced that it plans to progress these five new highly innovative drug candidates in the areas of oncology, cardiology, and women’s health into phase III clinical studies by 2015.
“Our Pharma research and development has done a tremendous job of bringing five new products to the market offering physicians and patients new treatment alternatives for serious diseases”, said Bayer CEO Dr. Marijn Dekkers. “Following our mission statement ‘Science For A Better Life’, the five chosen further drug candidates all have the potential to impact the way diseases are treated for the benefit of patients.”

Bayer CEO Dr. Marijn Dekkers
“Our research and development activities are strongly focused on areas where treatment options are not available today or where true breakthrough innovations are missing”, said Prof. Andreas Busch, member of the Bayer HealthCare Executive Committee and Head of Global Drug Discovery at Bayer HealthCare. “Our drug development pipeline holds a number of promising candidates which we want to bring to patients who need them urgently”, said Kemal Malik, member of the Bayer HealthCare Executive Committee, Chief Medical Officer and Head of Pharmaceutical Development at Bayer HealthCare. “Furthermore we are continuing to expand the range of indications for all our recently launched products Xarelto, Stivarga, Xofigo, Riociguat as well as Eylea and further refine the profile of these drugs in specific patient populations.”
“Our research and development activities are strongly focused on areas where treatment options are not available today or where true breakthrough innovations are missing”, said Prof. Andreas Busch, member of the Bayer HealthCare Executive Committee and Head of Global Drug Discovery at Bayer HealthCare. “Our drug development pipeline holds a number of promising candidates which we want to bring to patients who need them urgently”, said Kemal Malik, member of the Bayer HealthCare Executive Committee, Chief Medical Officer and Head of Pharmaceutical Development at Bayer HealthCare. “Furthermore we are continuing to expand the range of indications for all our recently launched products Xarelto, Stivarga, Xofigo, Riociguat as well as Eylea and further refine the profile of these drugs in specific patient populations.”
Cl 223Ra Cl
Xofigo
The five mid-stage candidates have been selected for accelerated development based on positive “proof-of-concept” data from early clinical studies. Three of them are development compounds in the area of cardiology or the cardio-renal syndrome: Finerenone (BAY 94-8862) is a next generation oral, non-steroidal Mineralocorticoid Receptor antagonist which blocks the deleterious effects of aldosterone. Currently available steroidal MR antagonists have proven to be effective in reducing cardiovascular mortality in patients with heart failure but have significant side effects that limit their utilization. Finerenone is currently in clinical Phase IIb development for the treatment of worsening chronic heart failure, as well as diabetic nephropathy.
Finerenone (BAY 94-8862)
The second drug candidate in the area of cardiology is an oral soluble guanylate cyclase (sGC) stimulator (BAY 1021189). The start of a Phase IIb study in patients with worsening chronic heart failure is expected later this year.
For the cardio-renal syndrome, a Phase IIb program with the investigational new drug Molidustat (BAY 85-3934) is under initiation in patients with anemia associated with chronic kidney disease and/or end-stage renal disease. Molidustat is a novel inhibitor of hypoxia-inducible factor (HIF) prolyl hydroxylase (PH) which stimulates erythropoietin (EPO) production and the formation of red blood cells. Phase I data have shown that inhibition of HIF-PH by Molidustat results in an increase in endogenous production of EPO.
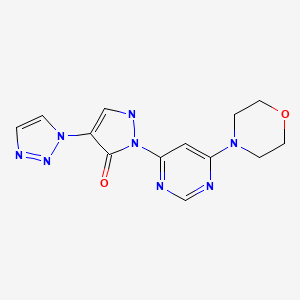
Molidustat (BAY 85-3934)
In oncology, Copanlisib (BAY 80-6946), a novel, oral phosphatidylinositol-3 kinases (PI3K) inhibitor, was selected for accelerated development. Copanlisib demonstrated a broad anti-tumor spectrum in preclinical tumor models and promising early clinical signals in a Phase I study in patients with follicular lymphoma. A Phase II study in patients with Non-Hodgkin’s lymphoma is currently ongoing.
Bayer has also made good progress in the development of new treatment options for patients with gynecological diseases: sPRM (BAY 1002670) is a novel oral progesterone receptor modulator that holds the promises of long-term treatment of patients with symptomatic uterine fibroids. Based on promising early clinical data the initiation of a Phase III study is planned for mid-2014.
Initiation of further studies with recently launched products
Bayer has successfully launched five new pharmaceutical products, namely Xarelto™, Stivarga™, Xofigo™, Eylea™, and Riociguat, which has very recently been approved in Canada under the trade name Adempas™.
Bayer has successfully launched five new pharmaceutical products, namely Xarelto™, Stivarga™, Xofigo™, Eylea™, and Riociguat, which has very recently been approved in Canada under the trade name Adempas™.


Regorafenib, stivarga
Bayer’s Eylea (aflibercept),
Xarelto has been approved globally for five indications across seven distinct areas of use, allowing doctors to treat patients in a greater variety of venous and arterial thromboembolic conditions than any other novel oral anticoagulant. The company continues to study the use of Xarelto for the treatment of further cardiovascular diseases. Ongoing clinical Phase III studies include COMPASS and COMMANDER-HF. The COMPASS study will assess the potential use of Xarelto in combination with aspirin, or as a single treatment to prevent major adverse cardiac events (MACE) in nearly 20,000 patients with atherosclerosis related to coronary or peripheral artery disease. The COMMANDER-HF study will evaluate the potential added benefit of Xarelto in combination with single or dual-antiplatelet therapy to help reduce the risk of death, heart attack and stroke in approximately 5,000 patients with chronic heart failure and coronary artery disease, following hospitalization for exacerbation of their heart failure.
In order to answer medically relevant questions for specific patient populations Bayer has initiated a range of additional Xarelto studies in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention with stent placement (PIONEER-AF-PCI), cardioversion (X-VERT) or an AF ablation procedure (VENTURE-AF).
As an extension to the Xarelto clinical trial programme, a number of real-world studies are designed to observe and further evaluate Xarelto in everyday clinical practice. These include the XAMOS study of more than 17,000 orthopaedic surgery patients, which confirmed the clinical value of oral, once-daily Xarelto in routine clinical practice in adults following orthopaedic surgery of the hip or knee. XANTUS is designed to collate data on real-world protection with Xarelto in over 6,000 adult patients in Europe with non-valvular AF at risk of stroke while XANAP is designed to collate data on real-world protection with Xarelto in over 5,000 adult patients in Europe and Asia with non-valvular AF at risk of stroke. XALIA will generate information from over 4,800 patients treated for an acute DVT with either Xarelto or standard of care.
In order to answer medically relevant questions for specific patient populations Bayer has initiated a range of additional Xarelto studies in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention with stent placement (PIONEER-AF-PCI), cardioversion (X-VERT) or an AF ablation procedure (VENTURE-AF).
As an extension to the Xarelto clinical trial programme, a number of real-world studies are designed to observe and further evaluate Xarelto in everyday clinical practice. These include the XAMOS study of more than 17,000 orthopaedic surgery patients, which confirmed the clinical value of oral, once-daily Xarelto in routine clinical practice in adults following orthopaedic surgery of the hip or knee. XANTUS is designed to collate data on real-world protection with Xarelto in over 6,000 adult patients in Europe with non-valvular AF at risk of stroke while XANAP is designed to collate data on real-world protection with Xarelto in over 5,000 adult patients in Europe and Asia with non-valvular AF at risk of stroke. XALIA will generate information from over 4,800 patients treated for an acute DVT with either Xarelto or standard of care.
In the area of oncology, Stivarga has been approved in 42 countries for use against metastatic colorectal cancer that is refractory to standard therapies, and additionally for gastrointestinal stromal tumor (GIST) in the US and Japan. Bayer is now planning to assess Stivarga in earlier stages of colorectal cancer as well as other cancer types. A Phase III trial in patients with colorectal cancer after resection of liver metastases is currently under initiation. Based on early clinical data Bayer has also initiated a Phase III study in liver cancer in patients who have progressed on sorafenib treatment.
Furthermore, the anti-cancer drug Xofigo (radium 223 dichloride) is a first-in-class alpha-pharmaceutical which is designed for use in prostate cancer patients with ‘bone metastases’ (secondary cancers in the bone) to treat the cancer in the bone and to help extend their lives. Xofigo is approved in the US for the treatment of patients with advanced castrate-resistant prostate cancer with symptomatic bone metastases. In addition, the European CHMP recently gave a positive opinion for radium 223 dichloride for the same use. The decision of the European Commission on the approval is expected in the fourth quarter of 2013.
Based on the excellent Phase III results for Xofigo in patients with castration resistant prostate cancer and symptomatic bone metastases Bayer is looking to expand the use of Xofigo to earlier stages of the disease, and plans to initiate a Phase III study in combination with the novel anti-hormonal agent abiraterone. In addition, early stage signal-generating studies in other cancer forms where bone metastases are important causes of morbidity and mortality are planned.
Based on the excellent Phase III results for Xofigo in patients with castration resistant prostate cancer and symptomatic bone metastases Bayer is looking to expand the use of Xofigo to earlier stages of the disease, and plans to initiate a Phase III study in combination with the novel anti-hormonal agent abiraterone. In addition, early stage signal-generating studies in other cancer forms where bone metastases are important causes of morbidity and mortality are planned.
In the area of pulmonary hypertension Adempas (Riociguat) is the first member of a novel class of compounds – so-called ‘soluble guanylate cyclase (sGC) stimulators’ – being investigated as a new and specific approach to treating different types of pulmonary hypertension (PH). Adempas has the potential to overcome a number of limitations of currently approved treatments for pulmonary arterial hypertension (PAH) and addresses the unmet medical need in patients with chronic thromboembolic pulmonary hypertension (CTEPH). It was approved for the treatment of CTEPH in Canada in September 2013, making it the world’s first drug approved in this deadly disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
In the area of ophthalmology, Eylea (aflibercept solution for injection) is already approved in Europe and several additional countries for the treatment of neovascular (wet) age-related macular degeneration and for macular edema following central retinal vein occlusion. In September, Bayer HealthCare and Regeneron Pharmaceuticals presented data of the two phase III clinical trials VIVID-DME and VISTA-DME of VEGF Trap-Eye for the treatment of diabetic macular edema (DME) at the annual meeting of the Retina Society in Los Angeles and at the EURetina Congress in Hamburg, Germany. Both trials achieved the primary endpoint of significantly greater improvements in best-corrected visual acuity from baseline compared to laser photocoagulation at 52 weeks. Bayer plans to submit an application for marketing approval for the treatment of DME in Europe in 2013.
About Bayer HealthCare
The Bayer Group is a global enterprise with core competencies in the fields of health care, agriculture and high-tech materials. Bayer HealthCare, a subgroup of Bayer AG with annual sales of EUR 18.6 billion (2012), is one of the world’s leading, innovative companies in the healthcare and medical products industry and is based in Leverkusen, Germany. The company combines the global activities of the Animal Health, Consumer Care, Medical Care and Pharmaceuticals divisions. Bayer HealthCare’s aim is to discover, develop, manufacture and market products that will improve human and animal health worldwide. Bayer HealthCare has a global workforce of 54,900 employees (Dec 31, 2012) and is represented in more than 100 countries. More information at www.healthcare.bayer.com.
The Bayer Group is a global enterprise with core competencies in the fields of health care, agriculture and high-tech materials. Bayer HealthCare, a subgroup of Bayer AG with annual sales of EUR 18.6 billion (2012), is one of the world’s leading, innovative companies in the healthcare and medical products industry and is based in Leverkusen, Germany. The company combines the global activities of the Animal Health, Consumer Care, Medical Care and Pharmaceuticals divisions. Bayer HealthCare’s aim is to discover, develop, manufacture and market products that will improve human and animal health worldwide. Bayer HealthCare has a global workforce of 54,900 employees (Dec 31, 2012) and is represented in more than 100 countries. More information at www.healthcare.bayer.com.

No comments:
Post a Comment