Andarine

(SARM-4, S-4), GTx-007
Acetamidoxolutamide
Androxolutamide
Androxolutamide
401900-40-1
WO 2002016310
Selective Androgen Receptor Modulators (SARM)
Signal Transduction Modulators
Andarine (GTx-007, S-4) is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for treatment of conditions such as muscle wasting, osteoporosis and benign prostatic hypertrophy, using the non-steroidal androgen antagonist bicalutamide as a lead compound.
Androxolutamide is a nonsteroidal selective androgen receptor modulator (SARM) which had been in early clinical trials at GTx for the treatment of cancer-related cachexia in several cancer types; however, no recent development has been reported for this indication. Preclinical studies had also been ongoing for the treatment of osteoporosis due to androgen deficiency in the aging male. The drug candidate is believed to bind to the testosterone receptor in such a way as to maximize the beneficial effects of the hormone like muscle growth, bone strengthening and enhanced libido, while minimizing the unwanted side effects, such as stimulation of prostate cancer, virilization and acne. This is accomplished by the selective modulation of the androgen receptor depending on tissue type.
The compound was originally developed at GTx. In March 2004, GTx entered into a joint collaboration and license agreement with Ortho Biotech, a wholly-owned subsidiary of Johnson & Johnson; however, in 2006 the agreement was terminated by mutual agreement of the companies.
Andarine is an orally active partial agonist for androgen receptors. It is less potent in both anabolic and androgenic effects than other SARMs. In an animal model of benign prostatic hypertrophy, andarine was shown to reduce prostate weight with similar efficacy to finasteride, but without producing any reduction in muscle mass or anti-androgenic side effects. This suggests that it is able to competitively block binding of dihydrotestosterone to its receptor targets in the prostate gland, but its partial agonist effects at androgen receptors prevent the side effects associated with the anti-androgenic drugs traditionally used for treatment of BPH
Family: Selective Androgen Receptor Modulator
Half Life: About 4 hours
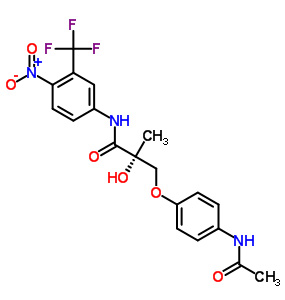
Formula: C19 H18 F3 N3 O6
Chemical Structure: S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide
Anabolic Rating: Similar to Testosterone Propionate
Facts: Ostarine (*S-4) is a Selective Androgen Receptor Modulator produced by GTx Inc, which is currently in the investigational stages of development. A SARM is exactly what it sounds like: a compound (not an anabolic steroid) which has the ability to stimulate the androgen receptor (much the same way as anabolic steroids). Unfortunately, due to its status as a drug still in the developmental stage, most of the research on it has been done in rodents and trials only.
S-4 is an orally active (and highly bioavailable) selective agonist for androgen receptors which was shown to have anabolic effects in muscle and bone tissue. It has been shown to have no measurable effect on lutenizing hormone (LH) or follicle-stimulating hormone (FSH), but it has been shown to have some effect on prostate weight, with an androgenic potency around 1/3rd of its anabolic potency (1). Still, this is a good trade-off, because it’s anabolic effect has been measured to be roughly the same as testosterone. It has also been shown to produce dose-dependent increases in bone mineral density and mechanical strength in addition to being able decrease body fat and increase lean body mass (2).
Unfortunately, it has a short half-life in humans of only 4 hours (3), and thus far has only gone through phase II clinical testing in humans (4).
Practical Use: This compound has potential use for all aspects of male hormone replacement therapy, and could eventually replace testosterone for this purpose. Since there is currently no accepted test for SARMs, athletes who are subject to drug testing would find it to be a suitable replacement for anabolic steroid use. Since it doesn’t effect LH or FSH, it may also be a highly useful anabolic agent to be used while attempting post-cycle therapy.
Side Effects: Prostate enlargement (1/3rd of what is seen with testosterone) and potential acne are potential side effects, although most users don’t report either of them; much more common are vision problems (floaters, yellow-tinged vision). Water retention, gynecomastia, and most other steroid-related side effects are probably not possible. In addition, inhibition of natural hormone levels is probably minimal or nonexistent at worst.
Producing/Developing Company:
Ostarine by GTx Inc.
References:
- Journal of Pharmacology And Experimental Therapeutics, Vol. 304, Issue 3, 1334-1340, March 2003
- Pharmaceutical Research. 2007 Feb;24(2):328-35.
- Pharmaceutical Research. 2006 Aug;23(8):1641-58.
- GTx Announces That Ostarine Achieved Primary Endpoint Of Lean Body Mass And A Secondary Endpoint Of Improved Functional Performance

The androgen receptor (′AR′″) is a ligand-activated transcriptional regulatory protein that mediates induction of male sexual development and function through its activity with endogenous androgens. Androgens are generally known as the male sex hormones. However, androgens also play a pivotal role in female physiology and reproduction. The androgenic hormones are steroids which are produced in the body by the testis and the cortex of the adrenal gland, or synthesized in the laboratory. Androgenic steroids play an important role in many physiologic processes, including the development and maintenance of male sexual characteristics such as muscle and bone mass, prostate growth, spermatogenesis, and the male hair pattern (Matsumoto, Endocrinol. Met. Clin. N. Am. 23:857-75 (1994). The endogenous steroidal androgens include testosterone and dihydrotestosterone (“DHT”) Testosterone is the principal steroid secreted by the testes and is the primary circulatiag androgen found in the plasma of males. Testosterone is converted to DHT by the enzyme 5 alpha-reductase in many peripheral tissues. DHT is thus thought to serve as the intracellular mediator for most androgen actions (Zhou, et al., Molec. Endocrinol. 9:208-18 (1995)). Other steroidal androgens include esters of testosterone, such as the cypionate, propionate, phenylpropionate, cyclopentylpropionate, isocarporate, enanthate, and decanoate esters, and other synthetic androgens such as 7-Methyl-Nortestosterone (“MENT′”) and its acetate ester (Sundaram et al., “7 Alpha-Methyl-Nortestosterone(MENT): The Optimal Androgen For Male Contraception,” Ann. Med., 25:199-205 (1993) (“Sundaram”)). Because the AR is involved in male sexual development and function, the AR is a likely target for effecting male contraception or other forms of hormone replacement therapy. The AR also regulates female sexual function (i.e., libido), bone formation, and erythropoiesis.
Worldwide population growth and social awareness of family planning have stimulated a great deal of research in contraception. Contraception is a difficult subject under any circumstances. It is fraught with cultural and social stigma, religious implications, and, most certainly, significant health concerns. This situation is only exacerbated when the subject focuses on male contraception. Despite the availability of suitable contraceptive devices, historically, society has looked to women to be responsible for contraceptive decisions and their consequences. Although health concerns over sexually transmitted diseases have made men more aware of the need to develop safe and responsible sexual habits, women still often bear the brunt of contraceptive choice. Women have a number of choices, from temporary mechanical devices such as sponges and diaphragms to temporary chemical devices such as spermicides. Women also have at their disposal more permanent options, such as physical devices like IUDs and cervical caps as well as more permanent chemical treatments, such as birth control pills and subcutaneous implants. However, to date, the only options available for men include the use of condoms or a vasectomy. Condom use, however is not favored by many men because of the reduced sexual sensitivity, the interruption in sexual spontaneity, and the significant possibility of pregnancy caused by breakage or misuse. Vasectomies are also not favored If more convenient methods of birth control were available to men, particularly long term methods that require no preparative activity immediately prior to a sexual act, such methods could significantly increase the likelihood that men would take more responsibility for contraception.
Administration of the male sex steroids (e.g., testosterone and its derivatives) has shown particular promise in this regard due to the combined gonadotropin-suppressing and androgen-substituting properties of these compounds (Steinberger et al, “Effect of Chronic Administration of Testosterone Enanthate on Sperm Production and Plasma Testosterone, Follicle Stimulating Hormones and Luteinizing Hormone Levels: A Preliminary Evaluation of a Possible Male Contraceptive”, Fertility and Sterility 28:1320-28 (1977)). Chronic administration of high doses of testosterone completely abolishes sperm production (azoospermia) or reduces it to a very low level (oligospermia). The degree of spermatogenic suppression necessary to produce infertility is not precisely known, However, a recent report by the World Health Organization showed that weekly intramuscular injections of testosterone enanthate result in azoospermia or severe oligospermia (i.e., less than 3 million sperm per ml) and infertility in 98% of men receiving therapy (World Health Organization Task Force on Methods Ar Regulation of Male Fertility, “Contraceptive Efficacy of Testosterone-Induced Azoospermia and Oligospermia in Normal Men,” Fertilily and Sterility 65:821-29 (1996)).
A variety of testosterone esters have been developed that are more slowly absorbed after intramuscular injection ancd, thus, result in greater androgenic effect. Testosterone enanthate is the most widely used of these esters. While testosterone enanthate has been valuable in terms of establishing the feasibility of hormonal agents for male contraception, it has several drawbacks, including the need for weekly injections and the presence of supraphysiologic peak levels of testosterone immediately following intramuscular injection (Wu, “Effects of Testosterone Enanthate in Normal Men: Experience From a Multicenter Contraceptive Efficacy Study,” Fertility and Sterility 65:626-36 (1996)).
“male drugs”. D. D. Miller, K. A. Veverka, and K. Chung report the large-scale synthesis of androgen-receptor modulators exemplified by 3a and 3b. These compounds have a variety of pharmaceutical applications related to male sex hormones, such as male contraceptives and drugs for treating prostate-related conditions. The inventors describe the kilogram-scale production of 3a and 3b by condensing 1 with 2a or 2b, as shown in Figure 1.

The reaction is carried out in the presence of a substantial excess of Cs2CO3 in THF. For the preparation of 3a, 6.17 mol Cs2CO3 is used with 3.37 mol 1; for 3b, 5.4 mol Cs2CO3and 2.7 mol 1 are used. (Disconcertingly, the patent shows the formula of the base as CsCO3, although the calculation of the molar amount is correct.) The preparation of 3atakes 3 h at 50 °C and is monitored by HPLC. TLC is used to monitor the synthesis of3b, which takes 8 h in refluxing THF.
To purify 3a, deionized water is added to an EtOH solution at room temperature to precipitate it; this process is repeated three times. The final yield of 3a is 83%. Purifying the product by using an alcohol and water is a key aspect of the patent and is covered in the claims. However, no analytical data are given to support the claimed purity. The workup of 3b also involves EtOH and water, but solvents EtOAc and MeO-t-Bu are also used; the product is isolated in 52% yield.
The inventors also describe the synthesis of compound 1 at kilogram scale (Figure 2). Acid chloride 5 is prepared by the reaction of carboxylic acid 4 with SOCl2. The acid chloride is not isolated, but it is treated with a solution of aniline derivative 6 and Et3N in THF over 3 h. After it is warmed to room temperature, the mixture is heated to 50 °C for 15 h. The reaction is monitored by TLC; 3.7 kg 1 is isolated by crystallization from warm toluene in 70.3% yield.

The multikilogram-scale synthesis of 4 is also described. The route, shown in Figure 3, starts with the preparation of compound 9 by simultaneously adding 4 M NaOH and a solution of acid chloride 8 in acetone to a mixture of carboxylic acid 7 and 4 M NaOH in acetone. The pH of the reaction mixture is kept at >10 by adding more 4 M NaOH as needed. Intermediate 9 is isolated by crystallization from MeO-t-Bu in 55.6% yield; it is then treated with N-bromosuccinimide (NBS) in DMF to cyclize it to 10. This is isolated in 87.7% yield by adding water to the reaction mixture. The final step is heating 10 to reflux in 24% aq HBr to produce 4, isolated as a crystalline solid from hot toluene in 81.3% yield.

The patent claims cover compounds related to 3a and 3b in which the nitro group is replaced by nitrile. Unfortunately, no examples are given describing the synthesis of these compounds. This is an efficient process for synthesizing 3a and 3b, and the inventors show that it is suitable for large-scale production. (University of Tennessee Research Foundation [Knoxville]. US Patent 7,968,721, June 28, 2011;

Novel pathway for the synthesis of arylpropionamide-derived selective androgen receptor modulator (SARM) metabolites of andarine and ostarine
TETRAHEDRON LETTERS,
TETRAHEDRON LETTERS,
Volume 54, Issue 18, Pages 2203-2282 (1 May 2013)
Pages 2239-2242
Katharina M. Schragl, Guro Forsdahl, Guenter Gmeiner, Valentin S. Enev, Peter Gaertner
Katharina M. Schragl, Guro Forsdahl, Guenter Gmeiner, Valentin S. Enev, Peter Gaertner

San Diego Rehab
ReplyDelete